Book traversal links for Summary of Evidence
The Statements and Recommendations on the use of hypofractionated radiotherapy for early (operable) breast cancer are based on two Cancer Australia systematic reviews:
- Cancer Australia systematic review of RCTs which included available evidence published from January 2001 to March 2010,19 that informed the clinical practice guidelines on the use of hypofractionated radiotherapy for early (operable) breast cancer published by Cancer Australia in November 2011.
- Updated Cancer Australia systematic review of RCTs to identify new and updated evidence published from January 2010 to November 2013.20
The primary search strategy was based on the 2011 systematic review. A total of 384 citations were identified, and following application of the exclusion criteria, six articles and three conference abstracts were identified for inclusion in the updated systematic review.
The total body of evidence on hypofractionated radiotherapy for early (operable) breast cancer from these two systematic reviews includes:
- Six primary Randomised Controlled Trials (RCTs): START A trial, START B trial, a trial by Spooner et al, the UK FAST trial, the Canadian trial and the United Kingdom Royal Marsden Hospital/Gloucestershire Oncology Centre (RMH/GOC) trial
- Three RCTs published as conference abstracts only.
Of the six primary RCTs, all but one (UK FAST) were included in the evidence base for the 2011 Cancer Australia Guidelines. Thus, the evidence base for the current guideline includes the most current data from these five trials together with data from the UK FAST trial.
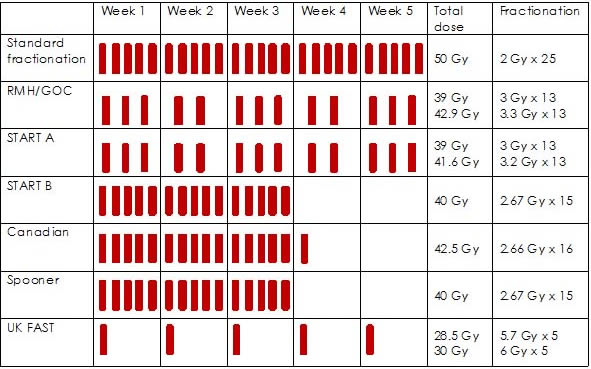
The RMH/GOC, START A and the UK FAST trials tested two hypofractionated radiotherapy regimens. The Canadian trial, the START B and Spooner trial each tested one hypofractionated radiotherapy regimen. In all trials, the conventional radiotherapy regimen used as a comparator was 50 Gy in 25 fractions, delivered over 5 weeks.
A range of hypofractionated radiotherapy regimens were examined, including:
- 39 Gy in 13 fractions over 35 days(RMH/GOC trial7,18 and START A10,16)
- 40 Gy in 15 fractions over 21 days (START B10,17 and Spooner trial11)
- 41.6 Gy in 13 fractions over 35 days (START A10,16)
- 42.5 Gy in 16 fractions over 22 days(Canadian trial6,13)
- 42.9 Gy in 13 fractions over 35 days(RMH/GOC trial 7,18)
- 30 Gy in 5 once weekly fractions of 6Gy over 5 weeks (UK FAST21)
- 28.5 Gy in 5 once weekly fractions of 5.7Gy over 5 weeks (UK FAST21)
Figure 1 provides a summary of hypofractionated radiotherapy regimens used in the RCTs. Four trials included women undergoing regional nodal radiotherapy: 14% in START-A; 7.3% in START-B; 20.6% in RMH/GOC and 100% in the Spooner trial.
Four trials included women who had undergone breast conserving surgery only (Spooner trial, UK FAST trial, Canadian trial, RMH/GOC).6,7,11,13,18,21 Two trials included women who had undergone breast conserving surgery or mastectomy (START A and START B).10,16,17
Median follow up ranged from 37.3 months in the UK FAST trial to 16.9 years in the Spooner trial.
Figure 1: hypofractionated radiotherapy schedules of RCTs22