Hypofractionated radiotherapy for early (operable) breast cancer
Hypofractionated radiotherapy for early (operable) breast cancer Anonymous (not verified)A CLINICAL PRACTICE GUIDELINE DEVELOPED BY CANCER AUSTRALIA
This guideline was first published in November 2011 and has been updated to incorporate new evidence.
This guideline includes statements, recommendations and practice points based on available, high-level evidence about the use of hypofractionated radiotherapy for the treatment of women with early (operable) breast cancer. The guideline aims to provide all health professionals within a multi-disciplinary team with information to assist in making management recommendations for improved patient outcomes.



Background
Background Anonymous (not verified)Early breast cancer is defined as tumours not more than five centimetres in diameter, with either impalpable lymph nodes or palpable but freely moveable lymph nodes, and with no evidence of distant metastases.1 Primary treatment of early breast cancer usually involves surgery to remove the tumour (breast conserving surgery or mastectomy) and management of the axilla.1 Complete pathology reporting following surgery will inform the adjuvant treatment options for individual women.
Several trials have shown that breast conserving surgery followed by whole breast radiotherapy is effective in reducing the risk of local recurrence and improving the long-term outcomes of appropriately selected patients with early breast cancer.2 Consequently, adjuvant radiotherapy is recommended for women who have undergone breast conserving surgery 1. Adjuvant chemotherapy may also be used in this patient population, but the circumstances of its use are beyond the scope of this guideline.
Conventional adjuvant whole breast radiotherapy is typically delivered over a period of 5 weeks using a standard dose of 2 Gray (Gy) per treatment episode (fraction) in 25 fractions to a total dose of 50 Gy.3 A tumour bed boost of 10-16 Gy in 2 Gy fractions 4,5 is sometimes delivered after whole breast radiotherapy.
Hypofractionated whole breast radiotherapy involves fewer fractions; however each fraction contains a larger daily dose of radiation than the conventional 2 Gy per fraction. The total dose of radiation used in a course of hypofractionated radiotherapy is reduced to compensate for the increased toxicity effect of larger daily fractions.
Compared to conventional radiotherapy regimens, the duration of a hypofractionated radiation treatment course is shorter by several days or weeks, as fewer fractions are required. A hypofractionated regimen may be more convenient for patients and less-resource intensive than a conventionally fractionated regimen. 6
Conventional radiotherapy and hypofractionated radiotherapy can be hypothesised to have a similar effect, based on radiobiological principles. The aim of hypofractionated radiotherapy is to balance as high a daily dose as possible in order to kill tumour cells, against a dose low enough to minimise the side-effects of treatment.
Sensitivity of tissues to radiation fraction size is described by the α/β ratio. Low α/β values indicate greater sensitivity to fraction size than higher α/β values. It has been hypothesised that breast cancer is as sensitive to fraction size as normal breast tissue with a low α/β value, and confirmation would indicate that fewer, larger fractions are as effective as conventional 2 Gy fractions.7
It is important to note that research on hypofractionated whole breast radiotherapy for early breast cancer is continuing. Clinical judgement should be applied in the context of the currently available evidence and emerging findings from the continuing body of research.
Grading of Clinical Practice Recommendations
Grading of Clinical Practice Recommendations Anonymous (not verified)The Recommendations are based on Statements of Evidence on the use of hypofractionated radiotherapy for the treatment of early (operable) breast cancer. Practice points are also provided to help guide clinical decisions for the use of hypofractionated radiotherapy for the treatment of early (operable) breast cancer. Practice points are based on expert opinion when the evidence to make a recommendation is insufficient or where the evidence is outside the scope of the systematic review.
All Recommendations have been graded using the National Health and Medical Research Council (NHMRC) FORM methodology.8,9 The NHMRC grades (A-D) assigned to the recommendation given are intended to indicate the strength of the body of evidence underpinning the recommendation (refer to Table 1). Appendix 1 provides further detail of the NHMRC FORM grading methodology and the process undertaken in the grading of all Recommendations contained in this guideline. See also Appendix 2 for Evidence Statements for Grading the Recommendations.
Table1: Definition of NHMRC grades of Recommendations8,9
| Grade of recommendation | Description |
|---|---|
|
A |
Body of evidence can be trusted to guide practice |
|
B |
Body of evidence can be trusted to guide practice in most situations |
|
C |
Body of evidence provides some support for recommendation(s) but care should be taken in its application |
|
D |
Body of evidence is weak and recommendation must be applied with caution |
Clinical Practice Recommendations and Practice Points
Clinical Practice Recommendations and Practice Points Anonymous (not verified)This clinical guideline is intended for all members of the multidisciplinary team responsible for the care of a woman with early breast cancer. Ideally, the recommendations regarding the use of different fractionation schedules should be considered prior to breast surgery.
Recommendations and practice points should be considered in the context of clinical judgement for each patient. Considerations should include the absolute benefits and harms of treatments, other treatments in use, patient preferences and quality of life issues. These factors should be discussed with the patient and their family or supporters, tailored to their preferences for information and decision-making involvement.
Patients
| Recommendations | Grade | References | |
|---|---|---|---|
|
1 |
In selected patients* with early breast cancer who require post-operative whole breast radiotherapy, hypofractionated radiotherapy is a suitable alternative to conventionally fractionated radiotherapy, and should be offered where appropriate. *Patients:
|
A |
Haviland 201310 (START A and B) Spooner 201211 UK FAST trial 201112 Whelan 20106 (Canadian trial) Owen 20067 (RMH/GOC trial)
|
|
2 |
For women outside the above criteria with early breast cancer who require post-operative whole breast radiotherapy, hypofractionated radiotherapy could be considered as an alternative to conventionally fractionated radiotherapy. Note: there is insufficient evidence to make a recommendation for or against the use of hypofractionated radiotherapy for men with breast cancer. |
C |
Haviland 201310 (START A and B) Spooner 201211 UK FAST trial 201112 Whelan 20106 (Canadian trial) Owen 20067 (RMH/GOC trial) |
|
Practice point |
|||
|
a |
Recent evidence indicates that tumour grade does not need to be taken into account when considering the use of hypofractionated radiotherapy |
Whelan 20106 Bane 201414 Haviland 201310 Herbert 201215 |
|
Optimal Schedules
| Recommendation | Grade | References | |
|---|---|---|---|
|
3 |
For women not receiving a tumour bed boost, recommended hypofractionated schedules for whole breast radiotherapy based on current evidence are:
|
A |
Haviland 201310 (START B) Spooner 201211
|
|
Practice point |
|||
|
b |
For women in whom a tumour bed boost is indicated, specific evidence-based dose-fractionation schedules for use with tumour bed boost have not been defined, but the following boost doses are considered acceptable:
|
Haviland 2013 (START B) 10
|
|
Adverse Events And Toxicity
| Recommendation | Grade | References | |
|---|---|---|---|
|
4 |
When selecting an appropriate radiotherapy schedule consideration should be given to the possibility of adverse events including acute reactions and late effects, noting that cosmetic outcomes are equivalent with the recommended optimal schedules for hypofractionated radiotherapy versus a conventionally fractionated radiotherapy schedule. |
B |
Haviland 201310 (START A and B) Spooner 201211 UK FAST trial 201112 Whelan 20106 (Canadian trial) Owen 20067 (RMH/GOC trial) |
|
Practice Point |
|||
|
c |
As cardiac effects from radiation therapy may take up to 20 years to develop, heart sparing protocols should be adopted irrespective of the dose fractionation regimen used. Particular consideration should be given to these effects when prescribing hypofractionated radiation therapy to the left breast, especially in women with pre-existing heart disease.. |
Haviland 201310 (START A and B)
|
|
Statements of Evidence
Statements of Evidence Anonymous (not verified)| No. | STATEMENTS OF EVIDENCE | Level of evidence | Reference |
|---|---|---|---|
|
In women with early (operable) breast cancer who have undergone total mastectomy: |
|||
| 1 |
There is insufficient evidence to inform the safety and efficacy of hypofractionated chest wall irradiation in women who have undergone mastectomy (total of 512 out of 8,367 (6%) patients across all studies). |
I and II |
Haviland 201310 (START A and B) Spooner 201211 UK FAST trial 201112 Whelan 20106 (Canadian trial) Owen 20067 (RMH/GOC trial) |
|
In women with early (operable) breast cancer who have undergone breast conserving surgery: |
|||
|
Patient and tumour characteristics |
|||
| 2 |
Hypofractionated radiotherapy is equivalent to conventionally fractionated regimens of radiotherapy in women aged over 50 years (79% of included patients), with pathological stage T1-2 (78% of included patients), N0 (75% of included patients), M0 breast cancer (100% of included patients) |
I and II |
Haviland 201310 (START A and B) Spooner 201211 UK FAST trial 201112 Whelan 20106 (Canadian trial) Owen 20067 (RMH/GOC trial) |
| 3 |
Due to the relatively small numbers of patients in sub-group analyses of randomised trials, there is only limited evidence to inform the safety and efficacy of hypofractionated radiotherapy for women:
|
II |
Haviland 201310 (START A and B) Spooner 201211 UK FAST trial 201112 Whelan 20106 (Canadian trial) Owen 20067 (RMH/GOC trial) |
|
Tumour grade |
|||
| 4 |
An unplanned sub-group analysis of the Canadian trial showed that for patients with high grade tumours, the hypofractionated radiotherapy regimen of 42.5 Gy in 16 fractions over 22 days was associated with a higher local recurrence rate compared with conventionally fractionated radiotherapy at 12 years follow-up (p=0.01). An updated analysis of the Canadian trial reported no statistically significant difference for local recurrence between grade 1-2 and grade 3 breast cancers (p=0.11). |
II
II |
Whelan 20106 (Canadian trial)
Bane 201414 |
| 5 |
A meta-analysis of the START A, START B trial and their pilot study reported no statistically significant difference in locoregional relapse between grade 1 and 2 tumours and grade 3 tumours (p=0.12). |
I |
Haviland 201310 |
| 6 |
A retrospective population based cohort study of patients with grade 3 breast cancer reported the 10-year cumulative incidence of local relapse was 6.9% in the hypofractionated group and 6.2% in the conventionally fractionated radiotherapy group (p=0.99). |
IV |
Herbert 201115 |
|
Optimal schedule |
|||
| 7 |
Two 3-week hypofractionated schedules, from three randomised controlled trials with 9.9 to16.9 years follow-up, demonstrated comparable rates of optimal tumour control and radiation therapy effects:
|
II |
Haviland 201310 (START B) Whelan 20106 (Canadian trial) Spooner 201211 |
|
Overall survival |
|||
| 8 |
No statistically significant difference in overall survival rates were reported for women treated with hypofractionated radiotherapy compared with patients treated with conventionally fractionated radiotherapy at 10-16.9 years follow-up. |
II |
Haviland 201310 (START A) Spooner 201211 Whelan 20106 (Canadian trial) |
| 9 |
One randomised controlled trial reported that a hypofractionated radiotherapy regimen of 40 Gy in 15 fractions over 21 days was associated with a statistically significant lower all-cause mortality, with up to 10 years follow-up, compared with conventionally fractionated radiotherapy; HR=0.80 (95% CI 0.65-0.99), p=0.042. |
II |
Haviland 201310 (START B)
|
|
Disease-free survival |
|||
| 10 |
One randomised controlled trial reported no significant difference in disease-free survival between the hypofractionated radiotherapy schedules and the conventionally fractionated radiotherapy regimen (41.6 Gy vs. 50 Gy HR=0.94, 95% CI 0.75-1.17, p=0.57; 39 Gy vs. 50 Gy HR=1.08, 95% CI 0.87-1.35, p=0.48). |
II |
Haviland 201310 (START A) |
| 11 |
One randomised controlled trial reported that a hypofractionated radiotherapy regimen of 40 Gy in 15 fractions over 21 days is associated with a statistically significant higher rate of disease-free survival than conventionally fractionated radiotherapy; HR=0.79 (95% CI 0.65-0.97), p=0.022. |
II |
Haviland 201310 (START B) |
|
Relapse-free survival |
|||
| 12 |
One randomised controlled trial reported no statistically significant difference in relapse-free survival between hypofractionated radiotherapy and conventionally fractionated radiotherapy; HR=0.98 (95% CI 0.75-1.29). |
II |
Spooner 201211 |
|
Local relapse |
|||
| 13 |
Five randomised trials reported no statistically significant difference in rates of local relapse for women treated with hypofractionated radiotherapy and conventionally fractionated radiotherapy at 9.7 to 16.9 years follow-up. |
II |
Haviland 201310 Spooner 201211 Whelan 20106 (Canadian trial) Owen 20067 (RMH/GOC trial) |
| 14 |
A hypofractionated radiotherapy regimen of 39 Gy in 13 fractions over 35 days is associated with a statistically significant higher rate of local recurrence compared with a hypofractionated radiotherapy regimen of 42.9 Gy in 13 fractions over 35 days at 10 years follow- up. |
II |
Owen 20067 (RMH/GOC trial) |
|
Local-regional relapse |
|||
| 15 |
Two randomised controlled trials reported no statistically significant difference in 10 year local-regional relapse rates between hypofractionated radiotherapy and conventionally fractionated radiotherapy. |
II |
Haviland 201310 (START A and START B) |
| 16 |
In a combined sub-group analysis of START A, START B and their pilot study there was no statistically significant difference in local-regional relapse rates between hypofractionated radiotherapy and conventionally fractionated radiotherapy by age, type of primary surgery, axillary node status, tumour grade, adjuvant chemotherapy use, or use of tumour bed boost radiotherapy. |
II |
Haviland 201310 (START A and START B) |
|
|
Distant relapse |
||
| 17 |
No statistically significant difference in distant relapse was reported in three randomised controlled trials between women receiving hypofractionated radiotherapy and patients receiving conventionally fractionated radiotherapy. |
II |
Haviland 201310 (START A) Spooner 201211 |
| 18 |
One randomised controlled trial reported that a hypofractionated radiotherapy regimen of 40 Gy in 15 fractions over 21 days is associated with a statistically significant lower rate of distant relapse up to 10 years follow-up, than conventionally fractionated radiotherapy; HR=0.74 (95% CI 0.59-0.94), p=0.014. |
II |
Haviland 201310 (START B) |
|
Adverse events |
|||
| 19 |
At 10 years follow-up, women receiving the hypofractionated radiotherapy regimens of 39 Gy in 13 fractions over 35 days (START A) and 40 Gy in 15 fractions over 21 days (START B) were statistically significantly less likely to experience moderate or marked breast shrinkage, telangiectasia, and breast oedema compared to the conventionally fractionated radiotherapy regimen. |
II |
Haviland 201310 (START A and START B) |
| 20 |
In a combined sub-group analysis of START A, START B and their pilot study the incidence of any moderate or marked physician-assessed normal tissue effects in the breast was not statistically significantly different between hypofractionated radiotherapy and conventionally fractionated radiotherapy irrespective of age, breast size, use of tumour bed boost radiotherapy, adjuvant chemotherapy, or tamoxifen. |
II |
Haviland 201310 (START A and START B) |
| 21 |
One randomised controlled trial reported that global cosmetic outcome worsened over time for women treated with either hypofractionated radiotherapy or conventionally fractionated radiotherapy, however there were no statistically significant differences observed over 10 years between the hypofractionated regimen of 42.5 Gy in 16 fractions over 22 days and the conventionally fractionated regimen. |
II |
Whelan 20106 (Canadian trial) |
| 22 |
One randomised controlled trial demonstrated a statistically significant dose response between 28.5 Gy in five once-weekly fractions of 5.7 Gy and 30 Gy in five once-weekly fractions of 6 Gy regimens, with worse results for change in photographic breast appearance at 2 years (p=0.002) in the 30 Gy patients and comparable rates for 28.5 Gy, compared with conventionally fractionated radiotherapy. |
II |
UK FAST trialists 201112 |
| 23 |
One randomised controlled trial reported that three-year rates of physician-assessed moderate/marked adverse effects in the breast were significantly higher in women receiving hypofractionated radiotherapy regimen of 30 Gy in 5 once weekly fractions of 6 Gy over 5 weeks compared with conventionally fractionated radiotherapy (p=<0.001) and the hypofractionated regimen of 28.5 Gy in 5 once weekly fractions of 5.7Gy over 5 weeks (p=<0.006). The rates were not statistically significantly different between the 28.5 Gy and 50 Gy groups. |
II |
UK FAST trialists 201112 |
| 24 |
One randomised controlled trial reported that the hypofractionated radiotherapy regimen of 39 Gy in 13 fractions over 35 days was associated with a lower risk of developing any late radiation effect than a conventionally fractionated radiotherapy regimen at 10 years follow-up. However, the hypofractionated regimen of 42.9 Gy in 13 fractions over 35 days was associated with a higher risk of developing any late radiation effect than a conventionally fractionated radiotherapy regimen at 10 years follow-up. |
II |
Owen 20067 (RMH/GOC trial) |
|
Cardiac toxicity |
|||
| 25 |
Although follow-up of 9.3 to 9.7 years is shorter than desired for late cardiac effects (i.e., 15-20 years), two randomised controlled trials observed no major difference between fractionation schedules for the number of women with left-sided primary tumours who subsequently experienced cardiac disease related death. |
II |
Haviland (2013) (START A and START B)
|
| 26 |
A third randomised controlled trial with only 3.1 years of follow-up observed no difference in the rates of cardiac disease related deaths for left- versus right-sided tumours. |
II |
UK FAST trialists 201112 |
|
Quality of life |
|||
| 27 |
No statistically significant differences in quality of life scores were found in women undergoing radiotherapy after surgery between hypofractionated and conventionally fractionated radiotherapy regimens at 5 years follow-up. |
II |
Bentzen 200816 (START A) Bentzen 200817 (START B) |
|
Regional nodal radiotherapy |
|||
| 28 |
There is insufficient evidence due to small or unreported sub-groups of patients in the included trials to support the use of hypofractionated regional nodal radiotherapy.
|
II |
Bentzen 200816 (START A) Bentzen 200817 (START B) Yarnold 200518 (RMH/COG trial) |
| 29 |
A four-field radiotherapy technique targeting the breast, ipsilateral axillary and supraclavicular lymph nodes was used in the Spooner study. At median follow-up of 16.9 years, hypofractionated radiotherapy was equivalent to conventionally fractionated radiotherapy. |
II |
Spooner 201211
|
|
|
Tumour bed boost |
||
| 30 |
In a post hoc combined sub-group analysis (n=5,861) of START A, START B and their pilot study, patients received a boost of 10 Gy in 5 fractions (planned before randomisation). There was no statistically significant difference in local-regional relapse rates nor moderate or marked physician-assessed normal tissue effects in the breast between hypofractionated radiotherapy and conventionally fractionated radiotherapy in patients who received tumour bed boost radiotherapy and those who did not receive tumour bed boost radiotherapy.
|
I |
Haviland 201310 (START A and START B, RMH/COG trial) |
| 31 |
All irradiated patients in the Spooner trial (n=358) received a supplementary boost to the tumour bed with a direct 10-14 mega electronvolt (MeV) electron field of 15 Gy in five daily fractions. At median follow-up of 16.9 years, hypofractionated radiotherapy was equivalent to conventionally fractionated radiotherapy, including tumour bed boost. |
II |
Spooner 201211
|
|
|
Chemotherapy/targeted therapies |
||
| 32 |
There is insufficient evidence due to small or unreported sub-groups of patients in the included trials to determine the safety and efficacy of hypofractionated radiotherapy for women who receive chemotherapy and/or targeted biological therapies. |
II |
Whelan 20106 (Canadian trial) Bentzen 200816 (START A) Bentzen 200817 (START B) Owen 20067 (RMH/COG trial) |
Summary of Evidence
Summary of Evidence Anonymous (not verified)The Statements and Recommendations on the use of hypofractionated radiotherapy for early (operable) breast cancer are based on two Cancer Australia systematic reviews:
- Cancer Australia systematic review of RCTs which included available evidence published from January 2001 to March 2010,19 that informed the clinical practice guidelines on the use of hypofractionated radiotherapy for early (operable) breast cancer published by Cancer Australia in November 2011.
- Updated Cancer Australia systematic review of RCTs to identify new and updated evidence published from January 2010 to November 2013.20
The primary search strategy was based on the 2011 systematic review. A total of 384 citations were identified, and following application of the exclusion criteria, six articles and three conference abstracts were identified for inclusion in the updated systematic review.
The total body of evidence on hypofractionated radiotherapy for early (operable) breast cancer from these two systematic reviews includes:
- Six primary Randomised Controlled Trials (RCTs): START A trial, START B trial, a trial by Spooner et al, the UK FAST trial, the Canadian trial and the United Kingdom Royal Marsden Hospital/Gloucestershire Oncology Centre (RMH/GOC) trial
- Three RCTs published as conference abstracts only.
Of the six primary RCTs, all but one (UK FAST) were included in the evidence base for the 2011 Cancer Australia Guidelines. Thus, the evidence base for the current guideline includes the most current data from these five trials together with data from the UK FAST trial.
The RMH/GOC, START A and the UK FAST trials tested two hypofractionated radiotherapy regimens. The Canadian trial, the START B and Spooner trial each tested one hypofractionated radiotherapy regimen. In all trials, the conventional radiotherapy regimen used as a comparator was 50 Gy in 25 fractions, delivered over 5 weeks.
A range of hypofractionated radiotherapy regimens were examined, including:
- 39 Gy in 13 fractions over 35 days(RMH/GOC trial7,18 and START A10,16)
- 40 Gy in 15 fractions over 21 days (START B10,17 and Spooner trial11)
- 41.6 Gy in 13 fractions over 35 days (START A10,16)
- 42.5 Gy in 16 fractions over 22 days(Canadian trial6,13)
- 42.9 Gy in 13 fractions over 35 days(RMH/GOC trial 7,18)
- 30 Gy in 5 once weekly fractions of 6Gy over 5 weeks (UK FAST21)
- 28.5 Gy in 5 once weekly fractions of 5.7Gy over 5 weeks (UK FAST21)
Figure 1 provides a summary of hypofractionated radiotherapy regimens used in the RCTs. Four trials included women undergoing regional nodal radiotherapy: 14% in START-A; 7.3% in START-B; 20.6% in RMH/GOC and 100% in the Spooner trial.
Four trials included women who had undergone breast conserving surgery only (Spooner trial, UK FAST trial, Canadian trial, RMH/GOC).6,7,11,13,18,21 Two trials included women who had undergone breast conserving surgery or mastectomy (START A and START B).10,16,17
Median follow up ranged from 37.3 months in the UK FAST trial to 16.9 years in the Spooner trial.
Figure 1: hypofractionated radiotherapy schedules of RCTs22
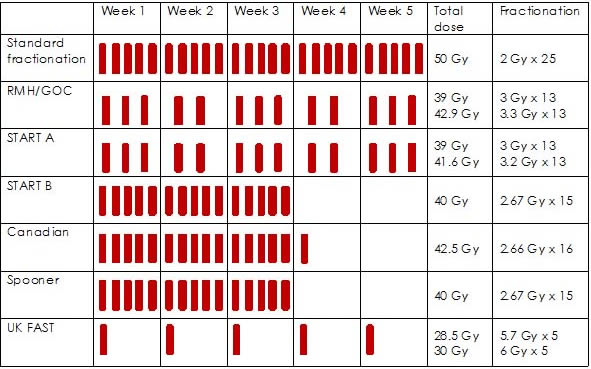
Characteristics of the Evidence Base
Characteristics of the Evidence Base Anonymous (not verified)Table 2 summarises the trial populations and primary outcomes measured in the six RCTs comparing hypofractionated radiotherapy to conventionally fractionated radiotherapy. Of note:
- Six trials identified the patient population characteristics as early invasive breast cancer T1-3, N0-1, M0.6,7,10,11,13,16-18,21
- The Spooner trial, UK FAST trial, the Canadian trial and the RMH/GOC, limited the trial populations to those who had breast conserving surgery only.6,7,11,13,18,21
- Women participating in the START A or START B trials had breast conserving surgery or mastectomy.10,16,17
Table 2 - Trial characteristics
| Trial | Population | Median follow-up (range) years | Intervention | Comparator | Outcomes measured | |
|---|---|---|---|---|---|---|
|
Studies Post Breast conserving surgery only |
||||||
|
Spooner 201211
|
Early breast cancer stage I and II |
16.9 (3.7-21.8) |
40 Gy in 15 daily fractions over 3wks (n=181) Supplementary boost of direct 10-14 MeV electron field of 15 Gy in five daily fractions |
50Gy in 25 fractions over 5 weeks (n=177) Supplementary boost of direct 10-14 MeV electron field of 15 Gy in five daily fractions |
Primary outcomes:
Secondary outcomes:
|
|
|
n=707 randomised to: radiotherapy (n=358) or no radiotherapy (n=349) |
||||||
|
UK FAST trial, 201121 |
Early stage breast cancer Tumour size <3.0cm |
3.1 |
30 Gy in 5 once weekly fractions of 6 Gy over 5 weeks (n=308) OR 28.5 Gy in 5 once weekly fractions of 5.7 Gy over 5 weeks (n=305) |
50 Gy in 25 fractions of 2 Gy over 5 weeks (n=302)
|
Primary outcomes:
Secondary outcomes:
|
|
|
Invasive carcinoma with negative axillary nodes |
12 (range NR) |
42.5 Gy in 16 fractions over 22 days (n=622) |
50 Gy in 25 fractions over 35 days (n=612) |
|
||
|
|
Early breast cancer, T1-3, N0-1, M0 <75 years
|
9.7 (7.8-11.8) |
39 Gy in 13 fractions over 5 weeks (n=474) 42.9 Gy in 13 fractions over 5 weeks (n=466) |
50 Gy in 25 fractions over 5 weeks (n=470) |
|
|
|
Post Breast conserving surgery or post mastectomy |
||||||
|
Early breast cancer T1-3a, N0-1, M0
|
9.3 |
39 Gy in 13 fractions over 5 weeks (n=737) OR 41.6 Gy in 13 fractions over 5 weeks (n=750) |
50 Gy in 25 fractions over 5 weeks (n=749) |
Primary outcomes:
Secondary outcomes:
|
||
|
Early breast cancer T1-3a, N0-1, M0
|
9.9 |
40 Gy in 15 fractions over 3 weeks (n=1110) |
50 Gy in 25 fractions over 5 weeks (n=1105) |
Primary outcomes:
Secondary outcomes:
|
||
Table 3 identifies key characteristics of patients involved in the six randomised controlled trials.
Table 3: Patient characteristics
| RMH/GOC7,18 | Canadian trial6,13 | START A10,16 | START B10,17 | Spooner 201211 | UK FAST trial21 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N=1410 | N=1234 | N=2235 | N=2215 | N=358 | N=915 | |||||||
|
|
n |
% |
n |
% |
n |
% |
n |
% |
n |
% |
n |
% |
|
Treated with breast conserving surgery |
1410 |
100% |
1234 |
100% |
1900 |
85% |
2038 |
92% |
358 |
100% |
915 |
100% |
|
Age ≥ 50 years |
987 |
70% |
929 |
75% |
1727 |
77% |
1758 |
79% |
|
|
915 |
100% |
|
T1-2 |
1383 |
98% |
904 |
73% |
1572 |
70% |
1667 |
75% |
173 |
48% |
812 |
89% |
|
N0 |
564 |
40% |
1234 |
100%23 |
1547 |
69% |
1635 |
74% |
358 |
100% |
915 |
100% |
|
N1 |
|
|
|
|
643 |
29% |
504 |
23% |
|
|
|
|
|
Adjuvant treatment None Tamoxifen Chemotherapy |
289 1074 196 |
21% 76% 14%
|
593 505 136 |
48% 41% 11% |
172 1758 793 |
8% 79% 35% |
84 1928 491 |
4% 87% 22% |
358 |
100% |
106 694 |
12% 76% |
|
High tumour grade |
|
|
233 |
19% |
629 |
28% |
509 |
23% |
62 |
17% |
98 |
11% |
Survival Results
Survival Results Anonymous (not verified)Overall survival
Four trials, START A, START B, Spooner 2012, and the Canadian trial, reported on overall survival. START B reported that the 10 year all-cause mortality rate was significantly lower in the hypofractionated radiotherapy arm than the standard radiotherapy arm; HR=0.80 (95% CI 0.65-0.99), p=0.042.10. The three other trials reported similar overall survival rates between hypofractionated radiotherapy and standard radiotherapy with no statistically significant differences.6,10,11
Disease-free survival
Both START A and START B reported disease-free survival (DFS). START B reported a significantly higher rate of DFS in patients receiving hypofractionated radiotherapy compared to standard radiotherapy; HR=0.79 (95% CI 0.65-0.97), p=0.022.10 Whereas START A reported no significant difference in DFS between the hypofractionated radiotherapy schedules and standard radiotherapy.10
Relapse-free survival
The trial by Spooner et al (2012) reported no significant difference between short- and long-course radiotherapy for relapse-free survival estimates at 2, 5, 10 and 15 years; HR=0.98; (95% CI 0.75-1.29), p-value not reported.11
Table 4: Survival outcomes of RCTs comparing hypofractionated radiotherapy and standard radiotherapy.
| Key outcomes | START A | START B | Spooner 2012 | Canadian trial | RMH/GOC trial |
|---|---|---|---|---|---|
|
Overall survival |
Equivalent 41.6 Gy HR=0.96, p=0.74; 39 Gy HR=1.05, p=0.69 |
Superior for hypofractionated radiotherapy HR=0.80, p=0.042 |
Equivalent HR=1.02, p-value NR, 95% CI NS |
Equivalent 10yr survival 84.6% hypofractionated vs. 84.4% control p=0.79 |
NR |
|
Disease-free survival |
Equivalent 41.6 Gy HR=0.94, p=0.57; 39 Gy HR=1.08, p=0.48 |
Superior for hypofractionated radiotherapy HR=0.79, p=0.022 |
NR |
NR |
NR |
|
Relapse-free survival |
NR |
NR |
Equivalent HR=0.98, p-value NR, 95% CI NS |
NR |
NR |
Relapse Results
Relapse Results Anonymous (not verified)Local Relapse
Five trials reported on local recurrence (START A, START B, Spooner 2012, RMH/GOC trial, Canadian trial). All trials reported similar rates of local relapse for women treated with hypofractionated radiotherapy and standard radiotherapy.6,7,10,11 See Tables 4 and 5. RMH/GOC noted a statistically significant difference in recurrence rates between the two hypofractionated regimens (42.9 Gy vs. 39 Gy: 9.6% vs. 14.8%, p=0.027) but not when either of the hypofractionated regimens was compared to 50 Gy in 25 fractions.7
Table 5: Five year rates for local recurrence rates in RMH/GOC and Canadian trials.
| Trial | Median follow-up (range) years | Treatment group | Five year local tumour recurrence rate (%) |
|---|---|---|---|
|
RMH/GOC7 |
9.7 (7.8-11.8) |
50 Gy in 25 fractions over 5 weeks |
12.1 |
|
42.9 Gy in 13 fractions over 5 weeks |
9.6 |
||
|
39 Gy in 13 fractions over 5 weeks |
14.8 |
||
|
Canadian6 |
12 (range not reported) |
50 Gy in 25 fractions over 5 weeks |
3.2^ |
|
42.5 Gy in 16 fractions over 22 days |
2.8^ |
||
|
Spooner 2012 |
16.9 (3.7-21.8) |
50 Gy in 25 fractions over 5 weeks |
9.6 |
|
40 Gy in 15 fractions over 3 weeks |
6.6 |
^6.7% and 6.2% at 10 years
Table 6: START A and START B relapses
| Events (n/patients; %) | Estimated proportion of patients with event by 5 years (%; 95% CI) | Estimated proportion of patients with event by 10 years (%; 95% CI) | Crude hazard ratio (95% CI) | P value | |
|---|---|---|---|---|---|
|
START A |
|||||
|
Local relapse |
|||||
|
50 Gy |
40/749 (5.3%) |
3.4% (2.3-5.1) |
6.7% (4.9-9.2) |
1.00 |
|
|
41.6 Gy |
37/750 (4.9%) |
3.1% (2.0-4.7) |
5.6% (4.1-7.8) |
0.90 (0.57-1.40) |
0.63 |
|
39 Gy |
47/737 (6.4%) |
4.4% (3.1-6.2) |
8.1% (6.1-10.7) |
1.20 (0.79-1.83) |
0.39 |
|
Local-regional relapse |
|||||
|
50 Gy |
45/749 (6.0%) |
4.0% (2.8-5.7) |
7.4% (5.5-10.0) |
1.00 |
|
|
41.6 Gy |
42/750 (5.6%) |
3.8% (2.6-5.5) |
6.3% (4.7-8.5) |
0.91 (0.59-1.38) |
0.65 |
|
39 Gy |
52/737 (7.1%) |
5.1% (3.7-7.1) |
8.8% (6.7-11.4) |
1.18 (0.79-1.76) |
0.41 |
|
Distant relapse |
|||||
|
50 Gy |
100/749 (13.3%) |
9.8% (7.9-12.3) |
14.7% (12.2-17.7) |
1.00 |
|
|
41.6 Gy |
110/750 (14.7%) |
9.5% (7.6-11.9) |
16.8% (14.0-20.0) |
1.08 (0.82-1.41) |
0.58 |
|
39 Gy |
121/737 (16.4%) |
11.8% (9.7-14.4) |
18.0% (15.1-21.2) |
1.24 (0.95-1.61) |
0.11 |
|
START B |
|||||
|
Local relapse |
|||||
|
50 Gy |
50/1105 (4.5%) |
3.3% (2.4-4.6) |
5.2% (3.9-6.9) |
1.00 |
|
|
40 Gy |
36/1110 (3.2%) |
1.9% (1.2-3.0) |
3.8% (2.7-5.2) |
0.70 (0.46-1.07) |
0.10 |
|
Local-regional relapse |
|||||
|
50 Gy |
53/1105 (4.8%) |
3.5% (2.5-4.8) |
5.5% (4.2-7.2) |
1.00 |
|
|
40 Gy |
42/1110 (3.8%) |
2.3% (1.5-3.4) |
4.3% (3.2-5.9) |
0.77 (0.51-1.16) |
0.21 |
|
Distant relapse |
|||||
|
50 Gy |
158/1105 (14.3%) |
10.5% (8.8-12.5) |
16.0% (13.8-18.5) |
1.00 |
|
|
40 Gy |
121/1110 (10.9%) |
7.5% (6.0-9.2) |
12.3% (10.3-14.6) |
0.74 (0.59-0.94) |
0.014 |
Local-regional relapse
Both START A and START B reported local-regional relapse rates. For both START A and START B there was no significant difference in 10 year local-regional relapse rates between hypofractionated radiotherapy and conventionally fractionated radiotherapy, see Table 5.10 In a combined sub-group analysis of START A, START B and their pilot study there was no significant difference in local-regional relapse rates between hypofractionated radiotherapy and conventionally fractionated radiotherapy by age, type of primary surgery, axillary node status, tumour grade, adjuvant chemotherapy use, or use of tumour bed boost radiotherapy.10
Distant relapse
START A, START B, and Spooner 2012 trials reported distant relapses.
START B reported the hypofractionated radiotherapy regimen to be associated with a statistically significant lower rate of distant relapse than standard radiotherapy; HR=0.74 (95% CI 0.59-0.94), p=0.014.10 See Table 5. Similar rates of distant relapse were reported between patients receiving hypofractionated radiotherapy and patients receiving conventionally fractionated radiotherapy in the START A, Spooner 2012 and UK FAST trials.10,11,21
Table 7: Recurrence outcomes of RCTs comparing hypofractionated radiotherapy and standard radiotherapy.
| Key outcomes | START A | START B | Spooner 2012 | Canadian trial | RMH/GOC trial |
|---|---|---|---|---|---|
|
Local recurrence |
Equivalent 41,6 Gy HR=0.90, p=0.63; 39 Gy HR=1.20, p=0.39 |
Equivalent HR=0.70, p=0.10 |
Equivalent HR and p-value NRc |
Equivalent 6.2% in hypofractionated vs. 6.7% in control at 10yrsd |
Equivalent hypofractionated vs. control. Superior for 42.9Gy vs. 39Gy 9.6% vs. 14.8% p=0.027 e |
|
Local-regional recurrence |
Equivalent 41.6 Gy HR=0.91, p=0.65; 39 Gy HR=1.18, p=0.41a |
Equivalent HR=0.77, p=0.21b |
NR |
NR |
NR |
|
Distant relapse |
Equivalent 41.6 Gy HR=1.08, p=0.58; 39 Gy HR=1.24, p=0.11 |
Superior for hypofractionated radiotherapy HR=0.74, p=0.014 |
Equivalent HR and p-value NR |
NR |
NR |
Yellow shaded cells indicate primary outcome for the trial.
a Target sample size 2000 patients to provide 80% power to detect a difference of 5%.
b Target sample size 1840 patients to provide 95% power to exclude an increase of 5% in local-regional relapse rate in the 40 Gy schedule compared to control.
c To detect a minimum of 10% excess in relapse in patients to radiotherapy or no radiotherapy (from 10 to 20% 5 year relapse rate) 300 patients in each treatment group were needed using 5% α level of significance and 90% power.
d The sample size for the trial, 600 patients per group, was based on earlier trial assumptions and a power of 80% with a one-sided alpha level of 5%.
e For an estimated 90% power and 5% significance level, 2250 patients would be needed to detect a 5% absolute increase in the risk of recurrence in either experimental group, compared with an expected 5-year local recurrence of 10% in the control group. Accrual was stopped before the target was reached, because this trial was superseded by the START trial, with tumour control as the primary endpoint.
Impact of tumour grade
Early evidence reported higher local recurrence rates for patients with high grade tumours. Recent analyses, as well as follow-up of the initial analysis, have demonstrated no significant difference for recurrence for high grade tumours.
Three studies examined hypofractionated radiotherapy in patients with high grade tumours; the Canadian trial and START trials as well as an additional retrospective population based cohort study by Herbert et al (2012).15
The 2010 publication of the Canadian trial by Whelan et al included an unplanned sub-group analysis including tumour grade.6 The analysis reported that for patients with high grade tumours, the cumulative incidence of local recurrence at 10 years was 15.6% in those receiving hypofractionated radiotherapy compared with 4.7% in those receiving conventional radiotherapy (p=0.01).6However, an updated analysis of the Canadian trial by Bane et al (2014) based on longer-term data, reported no statistically significant difference for local recurrence between grade 1-2 and grade 3 breast cancers (p=0.11).14
A meta-analysis of the START A, START B trial and their pilot study reported no significant difference in locoregional relapse between grade 1 and 2 tumours and grade 3 tumours (p=0.12).10
A retrospective population based cohort study by Herbert et al (2012) of patients with grade 3 breast cancer reported the 10-year cumulative incidence of local relapse was 6.9% in the hypofractionated group and 6.2% in the conventionally fractionated radiotherapy group (p=0.99).15
Safety Results
Safety Results Anonymous (not verified)Adverse events and cosmetic outcomes
Five trials reported on adverse events and cosmetic outcomes (START A, START B, UK FAST trial, RMH/GOC trial and the Canadian trial).
START A and START B trial results
Late normal tissue effects[*]
The most common normal tissue effects at 10 years were breast shrinkage and induration in both START trials. START A reported that in comparison to standard radiotherapy, patients in the 39 Gy regimen were significantly less likely to have moderate or marked breast induration, telangiectasia, and breast oedema.10 Moderate or marked normal tissue effects did not differ significantly between the hypofractionated radiotherapy regimen of 41.6 Gy and the 50 Gy group. In START B those receiving hypofractionated radiotherapy were significantly less likely to experience moderate or marked breast shrinkage, telangiectasia, and breast oedema compared to standard radiotherapy.10
Late adverse effects
For both START A and START B, ischaemic heart disease, symptomatic rib fracture and symptomatic lung fibrosis were rare at 10 years and incidence was similar between radiotherapy schedules.10
Change in breast appearance
START A reported that according to patient self-assessments of five normal tissue effects on the breast or breast area[†], the rates of moderate or marked effects at five years were similar for 41.6 Gy and 50 Gy.16 Rates of moderate or marked normal tissue effects tended to be lower after treatment in the 39 Gy group compared to the 50 Gy group, with a significantly lower rate of change in skin appearance (p=0.004). Changes in breast appearance and breast hardness were the most common changes reported.16
START A also measured change in breast appearance using photographic assessment; the hazard ratios for any change in breast appearance compared to the 50 Gy arm was 1.09 (p=0.62) after 41.6 Gy and 0.69 (p=0.01) after 39 Gy.16
Although mostly not statistically significant, the patient quality of life self-assessments of normal tissue effects in START B suggested that cosmetic outcomes were favourable in the 40 Gy group in most of the assessed normal tissue effects, with a significantly lower rate of change in skin appearance compared to the 50 Gy treatment arm (p=0.02).17 Changes in breast appearance and breast hardness were the most common changes reported. Photographic assessments also showed that change in breast appearance was less likely after treatment in the 40 Gy arm than the 50 Gy arm with a hazard ratio of 0.83 (p=0.06).17
Combined results of the START A and START B trials found that any change in skin appearance occurred significantly less often in the 39 Gy and 40 Gy arms when compared with the control arm of 50 Gy in 25 fractions over five weeks (39 Gy HR 0.63 95% CI 0.47-0.84, p=0.0019 and 40 Gy HR 0.76 95% CI 0.60-0.97, p=0.0262).24
UK FAST trial results
The UK FAST trial’s primary endpoint was change in photographic breast appearance measured by photographic assessments at baseline and at 2 years and 5 years.21 Assessments of 2-year change in photographic breast appearance were available for 81% of patients still alive and disease free. The trial reported the risk ratio for mild or marked change in 2 year photographic breast appearance for 30 Gy vs. 50 Gy was 1.70 (95% CI 1.26-2.29, p=<0.001) and for 28.5 Gy vs. 50 Gy the risk ratio was 1.15 (95% CI 0.82-1.60, p=0.489). The trial demonstrated a clear and statistically significant dose response between 28.5 Gy and 30 Gy with worse results for change in photographic breast appearance at 2 years in the 30 Gy patients. Outcomes were comparable between the 28.5 Gy schedule and 50 Gy schedule.21
Moderate or marked adverse effects in the breast were reported in 155 patients overall.21 Three-year rates of physician-assessed moderate/marked adverse effects in the breast were 17.3% (13.3-22.3%) for 30 Gy and 11.1% (7.9-15.6%) for 28.5 Gy compared with 9.5% (6.5-13.7%) after 50 Gy; the rate in the 30 Gy group was significantly higher than in 50 Gy (p=<0.001) and in 28.5 Gy (p=<0.006). The rates were similar between the 28.5 Gy and 50 Gy groups (p=0.18).21 Results for breast shrinkage were also significantly higher among patients in the 30 Gy group; 30 Gy vs. 50 Gy p=0.002 and 30 Gy vs. 28.5 Gy p=0.016 and similar between the 28.5 Gy and 50 Gy groups; p=0.455.
Canadian trial results
The Canadian trial reported on toxic effects of irradiation on the skin and subcutaneous tissue five and ten years after randomisation.6 The incidence of reported effects increased over the follow-up period, although the proportion of women with grade 3 radiation-associated skin and subcutaneous tissue morbidity was 4% or less, with no reports of grade 4 morbidity. At 10 years, there were no skin toxic effects for 70.5% of women in the conventional radiotherapy group, compared to 69.8% of women in the hypofractionated radiotherapy group. There were no toxic effects in subcutaneous tissue in 45.3% of women in the conventional radiotherapy group, compared with 48.1% of women in the hypofractionated radiotherapy group.6
Following assessments at baseline, three, five and ten years after randomisation, the global cosmetic outcome worsened over time however there were no significant differences observed between the 42.5 Gy group and the 50 Gy group at any time.6 At ten years follow-up, 71.3% of women in the 50 Gy group compared to 69.8% of women in the hypofractionated radiotherapy treatment group had an excellent or good cosmetic outcome.6 Cosmetic outcome was shown to be affected by time from randomisation, patient’s age and tumour size but there was no interaction with the treatment.6
RMH/GOC trial results
After a minimum follow-up of five years, the proportion of patients who recorded any change in breast appearance after 50 Gy in 25 fractions, 39 Gy in 13 fractions and 42 Gy in 13 fractions was 39.6%, 30.3% and 45.7% respectively.18
For photographically assessed changes in breast appearance, the trial found a higher risk of developing any radiation effect for patients allocated to 42.9 Gy in 13 fractions, compared to those allocated to 39 Gy in 13 fractions or 50 Gy in 25 fractions (p=<0.001 for comparison of three fractionation schedules).18
Clinical assessment of patients also indicated significant differences between the three fractionation schedules, with the 42.9 Gy group experiencing the highest incidence of events for overall breast cosmesis (p=<0.001), breast shrinkage (p=0.026), breast distortion (p=0.005), breast oedema (p=0.004), induration (p=0.001) and shoulder stiffness (p=0.001).18
Other adverse events
Three trials investigated the incidence of symptomatic lung fibrosis and symptomatic rib fracture.13,16,17 The reported rates were low at 5 years follow-up, and balanced between the regimens. One woman in the 41.6 Gy arm of the START A trial developed pneumonitis nine months after treatment; another developed mild signs of brachial plexopathy two years following treatment.16 The Canadian trial reported four cases of pneumonitis (two women in the 42.5 Gy group, and two women in the 50 Gy treatment group).13 One woman in the 50 Gy treatment group experienced rib fracture attributed to radiation therapy.13
While damage to the pectoral muscle has been highlighted as a possible concern,12 none of the trials reported this outcome
Table 8: Key cosmetic outcomes of RCTs comparing hypofractionated radiotherapy and standard radiotherapy
| Key outcomes | START A | START B | UK FAST trial | Canadian trial | RMH/GOC trial (Estimated % with no event at 10yrs) |
|---|---|---|---|---|---|
|
Physician assessed tissue effect* |
|||||
|
Overall |
NR |
NR |
Worse 30 Gy vs. 50 Gy: p=<0.001; Equivalent 28.5 Gy vs. 50 Gy: p=0.18 Superior in one hypofractionated schedule 30 Gy vs. 28.5 Gy: p=<0.006 |
NR |
NR |
|
Breast shrinkage |
Equivalent 41.6 Gy: HR 0.98, p=0.83 39 Gy: HR 0.86, p=0.19 |
Superior 40 Gy: HR 0.80, p=0.015 |
Worse 30 Gy vs. 50 Gy: p=0.002; Equivalent 28.5 Gy vs. 50 Gy: p=0.455 Superior in one hypofractionated schedule 30 Gy vs. 28.5 Gy: p=0.016 |
NR |
50 Gy: 36.2 42.6 Gy: 34.2 39 Gy: 44.4 p=0.026 |
|
Breast induration |
Superior 39 Gy: HR 0.76, p=0.034 Equivalent 41.6 Gy: HR 1.01, p=0.95 |
Equivalent 40 Gy: HR 0.81, p=0.084 |
Equivalent 30 Gy vs. 50 Gy: p=0.172; 28.5 Gy vs. 50 Gy: p=0.637 30 Gy vs. 28.5 Gy: p=0.323 |
NR |
50 Gy: 63.7 42.6 Gy: 48.9 39 Gy: 72.3 p=<0.001 |
|
Telangiectasia |
Superior 39 Gy: HR 0.43, p=0.003 Equivalent 41.6 Gy: HR 1.00, p=0.99 |
Superior 40 Gy: HR 0.62, p=0.032 |
NR |
NR |
50 Gy: 81.9 42.6 Gy: 82.0 39 Gy: 88.0 p=0.065 |
|
Breast oedema |
Superior 39 Gy: HR 0.54, p=0.001 Equivalent 41.6 Gy: HR 0.82, p=0.24 |
Superior 40 Gy: HR 0.55, p=0.001 |
NR |
NR |
50 Gy: 86.2 42.6 Gy: 78.5 39 Gy: 88.5 p=0.004 |
|
Shoulder stiffness |
Equivalent 41.6 Gy: HR 0.85, p=0.69 39 Gy: HR 0.74, p=0.49 |
Equivalent 40 Gy: HR 0.76, p=0.71 |
NR |
NR |
50 Gy: 90.0 42.6 Gy: 78.2 39 Gy: 89.9 p=<0.001 |
|
Arm oedema |
Equivalent 41.6 Gy: HR 1.31, p=0.45 39 Gy: HR 0.50, p=0.16 |
Equivalent 40 Gy: HR 0.42, p=0.21 |
NR |
NR |
50 Gy: 92.3 42.6 Gy: 89.5 39 Gy: 93.0 p=0.494 |
|
Breast distortion |
NR |
NR |
NR |
NR |
50 Gy: 41.5 42.6 Gy: 38.0 39 Gy: 51.4 p=0.005 |
|
Cosmesis (fair/poor) |
NR |
NR |
NR |
NR |
50 Gy: 28.8 42.6 Gy: 25.6 39 Gy: 42.0 p=<0.001 |
|
Other |
Equivalent 41.6 Gy: HR 1.09, p=0.79 39 Gy: HR 1.37, p=0.31 |
Superior 40 Gy: HR 0.65, p=0.018 |
NR |
NR |
NR |
|
Change in breast appearance |
|||||
|
Photographic assessed - Overall^ |
Superior 39 Gy: HR 0.69p=0.01 Equivalent 41.6 Gy: HR 1.09 p=0.62 |
Superior 40 Gy: HR 0.83 p=0.06 |
30 Gy vs. 50 Gy: RR 1.70 p=<0.001; 28.5 Gy vs. 50 Gy: RR 1.15 p=0.489 |
NR |
NR |
|
Photographic assessed - Any change in breast appearance |
NR |
NR |
NR |
NR |
50 Gy: 46.6 42.6 Gy: 42.0 39 Gy: 43.9 p=<0.001 |
|
Photographic assessed - Marked change in breast appearance |
NR |
NR |
NR |
NR |
50 Gy: 90.2 42.6 Gy: 84.4 39 Gy: 93.4 p=<0.001 |
|
Patient assessed# |
Superior 39 Gy: p=0.004 Equivalent 41.6 Gy: p= NR |
Superior 40 Gy: p=0.02 |
NR |
NR |
NR |
|
Global cosmetic outcome |
|||||
|
Global cosmetic outcome |
NR |
NR |
NR |
Equivalent 69.8% hypofract vs 71.3% control had excellent or good cosmetic outcome |
NR |
Cardiac toxicity
The 2013 Haviland article on the START trials reported on deaths from cardiac disease. In START A after 9.3 years median follow-up, 26/392 (6.6%) deaths were related to cardiac disease (seven with 50 Gy, 13 with 41.6 Gy, and six with 39 Gy). Fifteen (57.7%) of the 26 deaths from cardiac disease were in women with left-sided primary tumours (four of seven with 50 Gy, ten of 13 with 41.6 Gy, and one of six with 39 Gy). In START B, after 9.9 years median follow-up, 17/351 (4.8%) deaths were related to cardiac disease (12 with 50 Gy and five with 40 Gy). Eleven (64.7%) of the 17 deaths from cardiac disease were in women with left-sided primary tumours (eight of 12 with 50 Gy and three of five with 40 Gy). In UK FAST after 3.1 years median follow-up, 4/23 (17.4%) deaths were attributed to cardiac disease, with two deaths in women with left sided tumours, and two deaths in women with right-sided tumours. However, the UK FAST publication does not report the treatment group assignment for any of these cardiac disease related deaths.
In addition, while the Canadian trial did not report results for left- and right-sided breast cancers, the authors did note that at a median follow-up of 12 years few cardiac-related deaths were observed and no increase occurred in patients who received the hypofractionated regimen6.
When interpreting the mortality rates from START A, B and UK FAST a number of factors should be kept in mind. The number of events in each study is low and although women with pre-existing heart disease were excluded from START A and START B, none of the three studies stratified patients at baseline by cardiac risk factors. Furthermore, interpretation of the available evidence is potentially confounded by differences in the subsequent chemotherapy regimens administered to the women.
Haviland et al (2013) concluded that the START A and B trial results showed that although follow-up was still shorter than would be desired for cardiac events (i.e., 15-20 years 16, 17), there was no major difference between the fractionation schedules for the number of cases of heart disease in women with left-sided primary tumours.1 Haviland et al (2013) also note that the heart is sensitive to radiation whatever fractionation is used with no lower dose threshold for adverse effects. A commentary on the 2013 START trial results agreed with the START trial authors that techniques to protect the heart are important for both radiotherapy schedules and the choice of fractionation should not be affected by whether the tumour is in the left or right breast.25
Supplementary non-randomised trial evidence was also sourced on cardiotoxicity (refer to section on cardiotoxicity in technical document). A key population-based retrospective study by Chan et al was reported in two 2014 publications. The first (median follow-up 13.2 years; Ontario) determined if there is an increase in hospital-related morbidity from cardiac causes with either hypofractionated radiotherapy (40-44 Gy in 16 fractions) or conventional radiotherapy (45-50 Gy in 25 fractions or 50.4 Gy in 28 fractions).26 The second (median follow-up 14 years; Ontario) reported on if there is an increase in cardiac mortality with hypofractionated radiotherapy relative to conventional radiotherapy.27 Overall the authors concluded that for women with left-sided early-stage breast cancer who received postoperative radiation therapy to the whole breast or chest wall, there was no difference in the 15-year cardiac mortality or cumulative morbidity due to cardiac causes, between conventionally fractionated and hypofractionated treatment schedules.
Quality of life
Two trials reported quality of life outcomes using the European Organisation for Research and Treatment of Cancer (EORTC) breast cancer module.16,17 Three subscales were used in the analysis: breast symptoms (pain, swelling, oversensitivity, and skin problems in the breast); arm or shoulder symptoms subscale (swelling in the arm or hand, arm or shoulder pain, and difficulty moving the arm); and body image subscale. Based on these measures, there was no evidence that a hypofractionated radiotherapy regimen was associated with a statistically significant difference in quality of life scores.24 Sub-group analysis by surgery type was performed. The small numbers of patients and events in some sub-groups limited the statistical power of these analyses. There were no statistically significant differences in outcomes based on trial groups; nor were any interaction tests significant overall. 16,17
No other assessment of patient quality of life was available. Authors of the Canadian trial suggested that the inconvenience of a prolonged course of daily treatment made a substantial contribution to the decreased quality of life experienced by women treated with radiotherapy for breast cancer.13 A shorter fractionation schedule lessens the practical burden of treatment for women, and will have important quality of life benefits with respect to convenience and less time away from home and work.
Regional nodal radiotherapy
Regional nodal radiotherapy is the delivery of radiation to lymph nodes located in the breast region, namely the axillary and supraclavicular nodes on the same side as the affected breast. Four trials included women undergoing regional nodal radiotherapy (START A, START B, Spooner and RMH/GOC trial). None of these trials delivered radiation to the nodes of the internal mammary chain.
START A reported that the decision to administer regional nodal radiotherapy was made pre-randomisation and was used in approximately 14% of patients.16 One patient developed mild symptoms of brachial plexopathy but it was not reported if the patient received regional nodal radiotherapy. In two patients randomised to the 41.6 Gy arm and prescribed radiotherapy to the breast and supraclavicular fossa, the total dose was reduced to 39 Gy because of concerns regarding sensitivity of brachial plexus to fraction size.16
START B reported that 7.3% of patients received regional nodal radiotherapy.17 No cases of brachial plexopathy were reported among the women given radiotherapy to the supraclavicular fossa, axilla or both.17
Spooner et al (2012) reported that a four-field technique was used in all patients to irradiate the breast and ipsilateral axillary, and supraclavicular lymph nodes.11
RMH/GOC trial reported that 20.6% of patients underwent regional nodal radiotherapy to the axilla and/or supraclavicular fossa.18 There were no recorded cases of brachial plexopathy among these women.
[*] Normal tissue effects in the breast, arm, and shoulder were assessed by physician, photographic comparison with baseline, and patient self-reports.
[†] Patient quality of life self-assessments include the following changes since radiotherapy - breast shrinkage; breast hardness; change in skin appearance; swelling in area of affected breast; change in breast appearance.
Characteristics of Intervention
Characteristics of Intervention Anonymous (not verified)Use of tumour bed boost
Tumour bed boost was used in four of the randomised trials; START A, START B, Spooner 2012 and the RMH/GOC trial. Outcomes were reported for START A, START B and their pilot study, RMH/GOC, in a post hoc combined sub-group meta-analysis (n=5,861).10 Between January 1986 and July 1997, patients in the RMH/GOC trial were randomly assigned to receive a boost or not. Subsequently, all patients were offered an elective boost. The proportion of women who received a tumour bed boost was similar among the treatment groups. There was a statistically significant reduced risk of induration (p=0.001) and telangiectasia (p=0.026) in patients randomised to no boost.18
The proportion of women who received a tumour bed boost was similar among the treatment groups in the START A and START B trials. However, sub-group analysis on tumour bed boost was not reported.16,17 In a combined sub-group analysis of START A, START B and their pilot study there was no statistically significant difference in local-regional relapse rates or moderate or marked physician-assessed normal tissue effects in the breast between hypofractionated radiotherapy and conventionally fractionated radiotherapy in patients who received tumour bed boost radiotherapy and those who did not receive tumour bed boost radiotherapy (Haviland 2013). This analysis provides support of equivalence between hypofractionated radiotherapy with boost and conventional radiotherapy without boost for tumour control and improved late tissue effects, with the inclusion of 5,861 patients and lengthy follow-up. While the 2011 ASTRO guidelines state “there were few data to define the indications for and toxicity of a tumour bed boost in patients treated with hypofractionated radiotherapy” this guideline was published in 2011, prior to the 2013 START trial publication of tumour bed boost data.
In the Spooner et al trial (2012), all irradiated patients received a supplementary boost to the local tumour site of a direct 10-14 MeV electron field of 15 Gy in five daily fractions.11
Delivery of radiotherapy
All trials provided information on the radiotherapy techniques used. Patients in all six trials were treated in a supine position. The RMH/GOC and Canadian trials specified that patients were treated with one or both arms raised above the shoulder and Spooner noted patients had arm abducted to 900.
In five trials, 6-megavoltage x-rays were used for most patients but higher energy megavoltage x-rays or cobalt x-rays were also used.6,7,13,16-18,21 Where regional radiotherapy was indicated, the target volume included the supraclavicular nodes with or without the axillary nodes.7,16,17
Four trials reported that the maximum dose to the breast on the central axis was no less than 93% to 95% and no more than 105% to 107% of the prescribed dose.6,7,13,16-18. The Canadian trial excluded patients whose separation along the central axis exceeded 25cm; however the other trials used higher energy x-rays for patients with larger breasts to achieve acceptable dose homogeneity.6,7,13,16,17 RMH/GOC and Canadian trials reported the use of wedge tissue compensators to ensure a uniform dose distribution throughout the target volume.7,13,18
Four trials included women allocated to receive a tumour bed boost. Women allocated to receive a boost in RMH/GOC received a dose of 14 Gy to the 90% isodose (15.5 Gy to 100%) in 7 daily fractions.18 Ten Gy in 5 daily fractions to the 100% isodose was delivered after whole breast radiotherapy to women allocated to receive a boost in the START A and START B trials.16,17 All irradiated patients in the Spooner trial received a supplementary boost to the local tumour site of a direct 10-14 MeV electron field of 15 Gy in five daily fractions.11
Use of adjuvant systemic therapies
Five trials included women who received adjuvant systemic therapies; START A, START B, Spooner 2012, the Canadian trial and RMH/GOC trial. In the Canadian trial, 11% of women received chemotherapy in both the conventional and hypofractionated radiotherapy regimens; and 41% received tamoxifen in both the conventional and hypofractionated radiotherapy regimens.6 Sub-group analysis of the rates of local recurrence showed no statistically significant difference between the conventional and hypofractionated regimens at five years and ten years.6
No sub-group analysis on the use of systemic therapies was reported in the RMH/GOC, START A or START B trials or Spooner trial.7,16-18 In each trial, the proportions of women who received systemic therapies including tamoxifen and/or chemotherapy were similar among the study groups. The START trials required a two week gap between exposure to chemotherapy and radiotherapy.16,17 In the Spooner trial the authors noted that the study was conducted at a time when few patients were given adjuvant chemotherapy and all patients received tamoxifen because hormone receptor status was not routinely available. Patients in the trial received tamoxifen (20mg once daily) for a minimum of 2 years, after which there was a subsequent sub-randomisation to discontinue or continue for at least another 3 years.11
No trials specifically assessed the use of hypofractionated radiotherapy in conjunction with chemotherapy or other biological therapies.
Strengths and Weaknesses of the Evidence
Strengths and Weaknesses of the Evidence Anonymous (not verified)Overall the evidence included in the systematic review was based on six randomised controlled trials which were considered to be of high quality.
All trials were randomised, with the methods of randomisation considered high quality. The trials were open label and not blinded. Survival outcomes by intention-to-treat analysis were reported by most trials and limited numbers of patients were lost to follow-up (less than 5%). All trials had standardised assessment of outcomes and had well matched population characteristics between treatment arms at baseline.
All reported outcomes need to be considered in the context of the range of hypofractionated radiotherapy regimens that were evaluated. Although 50 Gy in 25 fractions was used as a control arm in all trials, seven different hypofractionated radiotherapy regimens were investigated.
Unanswered questions
Unanswered questions Anonymous (not verified)Important unanswered questions about the use of hypofractionated radiotherapy in early breast cancer are outlined below. Some of these questions may be addressed in ongoing trials:
- Treatment outcomes for patients who received hypofractionated radiotherapy in relation to age and tumour size.
- Optimal hypofractionated radiotherapy schedule.
- Safety and efficacy of different tumour bed boost protocols administered after hypofractionated radiotherapy.
- Safety and efficacy of hypofractionated regional nodal radiotherapy.
- Hypofractionated radiotherapy for DCIS.
- Potential interactions between adjuvant systemic therapies and hypofractionated radiotherapy.
- Long-term effects of hypofractionated radiotherapy on cardiac toxicity.
- Long-term effects of hypofractionated radiotherapy on rib morbidity.
- Psychosocial outcomes for women receiving hypofractionated radiotherapy, including impact of hypofractionated radiotherapy on quality of life, such as side-effects and practical implications of a shorter treatment schedule.
- Health economic considerations of hypofractionated radiotherapy.
Ongoing trials
Ongoing trials Anonymous (not verified)The following randomised controlled trials are investigating the use of hypofractionated radiotherapy for early breast cancer:
- NCT0000156130 trial compares 42.5 Gy in 16 fractions over 22 days with 50 Gy in 25 fractions over 35 days in patients diagnosed with early (invasive) breast cancer followed by breast conserving surgery or mastectomy.
- NCT01349322 trial compares accelerated hypofractionated radiotherapy with a concurrent boost 5 days a week for 3 weeks, with standard whole-breast radiotherapy for a 5 days a week for 3-5 weeks followed by sequential radiotherapy boost, in patients diagnosed with early stage breast cancer removed by surgery. Accelerated fractionation refers to schedules where the dose per fraction is unchanged but the daily dose is increased and the total treatment time is reduced.
- NCT00005587 trial compares patients receiving radiotherapy 5 times a week for 3 weeks for a total dose of 40 Gy for patients with microscopic evidence of invasive or in situ cancer at, or within 1mm of, a resection margin receive radiotherapy for 5 fractions in 1 week for a total boost of 10 Gy, with patients receiving a control dose of 50 Gy in 25 fractions over 5 weeks. All patients were diagnosed with early stage breast cancer removed by local excision or mastectomy.
- ‘Fast-forward’ trial compares 27 Gy or 26 Gy in five fractions over 5 days, with a control dose of 40 Gy in 15 fractions over 15 days in patients diagnosed with invasive carcinoma of the breast removed by breast conservation surgery.
- ‘SHARE’ trial compares 42.5 Gy in 16 fractions or 40 Gy in 15 fractions over 3 weeks or 40 Gy in 10 fractions over 3 weeks, with a control dose of 50 Gy in 25 fractions over 35 days followed by a 10 to 16 Gy boost in 5 to 8 fractions. All patients were diagnosed with invasive carcinoma of the breast.
International guidelines
International guidelines Anonymous (not verified)The following international guidelines have been identified which include guidance on the use of hypofractionated radiotherapy for early breast cancer:
- The American Society for Radiation Oncology (ASTRO) guidelines on fractionation for whole breast irradiation, 2010
- The New Zealand Ministry of Health Guidelines for Management of Early Breast Cancer, 2009
- NICE Guidelines for early and locally advanced breast cancer, 2009
- Scottish Intercollegiate Guidelines Network (SIGN) guidelines, 2009
- BC Cancer Agency Breast cancer management consensus guidelines 2013
- European Journal of Medical Oncology (ESMO) guidelines on primary breast cancer diagnosis, treatment and follow-up, 2013
- German Society of Radiation Oncology (DEGRO) guidelines on radiotherapy of breast cancer, 2013
- Nice-Saint-Paul de Vence guidelines on adjuvant radiotherapy in the management of axillary node negative invasive breast cancer, 2013
The BC Cancer Agency consensus based guidelines for the management of early breast cancer include recommendations on the use of radiotherapy and recommend a hypofractionated radiotherapy regimen as standard. The guideline recommends the following dose fractionation for radiotherapy following breast conserving therapy (T1, T2; N0):
- Standard whole breast dose is 42.5 Gray (Gy) in 16 daily fractions
- Certain patients are at risk for inferior cosmetic outcome from the 16-fraction course. Extended fractionation should be considered for patients with very large breast size, and those with significant post-operative induration, oedema, erythema, hematoma or infection. Patients with these indications for extended fractionation should receive 45Gy in 25 daily fractions plus a boost dose of 10Gy in 5 fractions or 50.4 Gy in 28 daily fractions.
- If a boost is used, an additional dose of 6-16 Gy in 3-8 fractions is recommended.
The guideline recommends the following dose fractionation following mastectomy or BCS (T1,T2; N1, and T3;N0):
- Standard whole breast dose is 42.5 Gy in 16 daily fractions, chest wall dose is 40 Gy in 16 fractions, nodal dose is 37.5-40 Gy/16 fractions.
- Those at risk for increased toxicity post-BCS should be treated with the breast doses described above in the T1, T2, N0 section. Nodal dose should be 45 Gy/25 fractions.
- Those at risk for increased toxicity post-mastectomy, e.g. postoperative infection, and those undergoing reconstruction post-mastectomy should also be considered for extended fractionation. Patients with indications for extended fractionation post-mastectomy should receive 50.4 Gy in 28 daily fractions to the chest wall, and 45 Gy in 25 fractions to the nodal regions.
- For those with close or positive margins post-mastectomy, a higher chest wall dose (e.g. 42.5-44 Gy in 16 fractions) may be used, or a boost dose of 10Gy in 4 fractions or 16Gy in 8 fractions may be considered, if the anatomic area requiring the boost dose can be accurately delineated.
References
References Anonymous (not verified)- National Breast Cancer Centre. Clinical practice guidelines for the management of early breast cancer (2nd edition). Commonwealth of Australia, Canberra, 2001.
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Darby S, McGale P, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707-16.
- James ML, Lehman M, Hider PN, et al. Fraction size in radiation treatment for breast conservation in early breast cancer. Cochrane Database Syst Rev. 2008;3):CD003860.
- Faculty of Radiation Oncology and The Royal Australian and New Zealand College of Radiologists. RANZCR The Clinicians guide to radiation oncology. M Barton, Editor, Sydney, 2002.
- Poortmans PM, Collette L, Bartelink H, et al. The addition of a boost dose on the primary tumour bed after lumpectomy in breast conserving treatment for breast cancer. A summary of the results of EORTC 22881-10882 "boost versus no boost" trial. Cancer Radiother. 2008;12(6-7):565-70.
- Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362(6):513-20.
- Owen JR, Ashton A, Bliss JM, et al. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: long-term results of a randomised trial. Lancet Oncol. 2006;7(6):467-71.
- Hillier S, Grimmer-Somers K, Merlin T, et al. FORM: An Australian method for formulating and grading recommendations in evidence-based clinical guidelines. BMC Medical Research Methodology. 2011;11(23):11-23.
- National Health and Medical Research Council (NHMRC). NHMRC additional levels of evidence and grades for recommendations for developers of guidelines. NHMRC, Commonwealth of Australia, 2009.
- Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14(11):1086-94.
- Spooner D, Stocken DD, Jordan S, et al. A Randomised Controlled Trial to Evaluate both the Role and the Optimal Fractionation of Radiotherapy in the Conservative Management of Early Breast Cancer. Clinical Oncology. 2012;24(10):697-706.
- Yarnold J, Bentzen SM, Coles C and Haviland J. Hypofractionated whole-breast radiotherapy for women with early breast cancer: myths and realities. Int J Radiat Oncol Biol Phys. 2011;79(1):1-9.
- Whelan T, MacKenzie R, Julian J, et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J Natl Cancer Inst. 2002;94(15):1143-50.
- Bane AL, Whelan TJ, Pond GR, et al. Tumor Factors Predictive of Response to Hypofractionated Radiotherapy in a Randomized Trial Following Breast Conserving Therapy. Ann Oncol. 2014;.
- Herbert C, Nichol A, Olivotto I, et al. The Impact of Hypofractionated Whole Breast Radiotherapy on Local Relapse in Patients with Grade 3 Early Breast Cancer: A Population-based Cohort Study. Int J Radiat Oncol Biol Phys. 2012;82(5):2086-92.
- Bentzen SM, Agrawal RK, Aird EG, et al. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol. 2008;9(4):331-41.
- Bentzen SM, Agrawal RK, Aird EG, et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet. 2008;371(9618):1098-107.
- Yarnold J, Ashton A, Bliss J, et al. Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: long-term results of a randomised trial. Radiother Oncol. 2005;75(1):9-17.
- National Breast and Ovarian Cancer Centre. A systematic literature review of hypofractionated radiotherapy for the treatment of early breast cancer. NBOCC, Surry Hills NSW, 2010.
- Cancer Australia. Hypofractionated radiotherapy for the treatment of early breast cancer: an updated systemantic review. Cancer Australia, Surry Hills, 2014.
- FAST Trialists group, Agrawal RK, Alhasso A, et al. First results of the randomised UK FAST Trial of radiotherapy hypofractionation for treatment of early breast cancer (CRUKE/04/015). Radiother Oncol. 2011;100(1):93-100.
- Fisher CM and Rabinovitch R. Frontiers in radiotherapy for early-stage invasive breast cancer. J Clin Oncol. 2014;32(26):2894-901.
- Smith BD, Bentzen SM, Correa CR, et al. Fractionation for Whole Breast Irradiation: An American Society for Radiation Oncology (ASTRO) Evidence-Based Guideline. Int J Radiat Oncol Biol Phys. 2011;81(1):59-68.
- Hopwood P, Haviland JS, Sumo G, et al. Comparison of patient-reported breast, arm, and shoulder symptoms and body image after radiotherapy for early breast cancer: 5-year follow-up in the randomised Standardisation of Breast Radiotherapy (START) trials. Lancet Oncol. 2010;11(3):231-40.
- Haffty BG and Buchholz TA. Hypofractionated breast radiation: preferred standard of care? Lancet Oncol. 2013;14(11):1032-4.
- Chan EK, Woods R, McBride ML, et al. Adjuvant hypofractionated versus conventional whole breast radiation therapy for early-stage breast cancer: long-term hospital-related morbidity from cardiac causes. Int J Radiat Oncol Biol Phys. 2014;88(4):786-92.
- Chan EK, Woods R, Virani S, et al. Long-term mortality from cardiac causes after adjuvant hypofractionated vs. conventional radiotherapy for localized left-sided breast cancer. Radiother Oncol. 2014.
Acknowledgements
Acknowledgements Anonymous (not verified)Membership of Hypofractionated Radiotherapy Working Group
In 2014, the update was overseen by a multidisciplinary working group convened by Cancer Australia:
- Dr Marie-Frances Burke (Chair) - Radiation Oncologist
- Ms Jan Rice - Breast care nurse
- Dr Kirsty Stuart - Radiation Oncologist
- Dr Patsy Soon - Breast Surgeon
- Ms Bronwyn Wells - Consumer representative
The orginal guideline was developed by a multidisciplinary working group convened by NBOCC [1].
- A/Prof Boon Chua (Chair) - Radiation Oncologist
- Dr Marie-Frances Burke - Radiation Oncologist
- Prof Geoff Delaney - Radiation Oncologist
- Dr Jane O’Brien - Breast Surgeon
- Ms Jan Rice - Breast Care Nurse
- Ms Geraldine Robertson - Consumer Representative
- Dr Kirsty Stuart - Radiation Oncologist
Topic-specific guideline development process
Priority topic areas for guideline development are determined in consultation with key stakeholders including experts in relevant disciplines and consumer representatives. A specific multidisciplinary Working Group, including consumers, is established for each topic identified and is involved in all aspects of guideline development. A systematic evidence review is undertaken for each guideline. All members are asked to declare any conflicts of interest and these declarations are recorded. The content of the guideline is not influenced by any external funding body. The guideline is reviewed externally by key stakeholders and the wider community and endorsement is sought from relevant professional colleges and groups in Australia.
[1] In July 2011, NBOCC amalgamated with Cancer Australia to form a single national agency, Cancer Australia, to provide leadership in cancer control and improve outcomes for Australians affected by cancer.
Appendix 1: Grading The Recommendations
Appendix 1: Grading The Recommendations Anonymous (not verified)Grading methodology
To accurately assess the strength of evidence available, the NHMRC methodology (FORM) was used in this clinical practice guideline to grade recommendations. The aim of this approach by NHMRC is to assist clinical practice guideline developers with a structured process for evaluating the evidence base corresponding to a particular key clinical question, in the context of the setting in which it is to be applied.8
The grading methodology allows for both the quality of the evidence and the strength of recommendations to be determined. Where insufficient evidence exists to formulate a grade, a practice point may be assigned instead. The NHMRC grading framework allows for these practice points to be included when developers consider it is important to provide non-evidence-based guidance.8
The NHMRC Evidence Statement Form sets out the basis for rating five key components of the ‘body of evidence’ for each recommendation. These components are:
- The evidence base, in terms of the number of studies, level of evidence and quality of studies (risk of bias).
- The consistency of the study results
- The potential clinical impact of the proposed recommendation
- The generalisability of the body of evidence to the target population for the guideline
- The applicability of the body of evidence to the Australian healthcare context9.
The first two components describe the internal validity of the study data in support of efficacy (for an intervention), accuracy (for a diagnostic test), or strength of association (for a prognosis or aetiological question). As suggested, the third component gives the likely clinical impact of the proposed recommendation. The final two components assess external factors that may influence the effectiveness of the proposed recommendation in practice, in terms of generalisability of study results to the intended target population for the Guideline and setting of the proposed recommendation, and applicability to the Australian (or other local) health care system.9
These described components should be rated according to the body of evidence matrix (refer to Table 9). The matrix system is used to summarise the rating of the five key components which allows each recommendation to be assigned an overall NHMRC Grade of Recommendation (A-D).8
Table 9: NHMRC Body of evidence matrix9
| Component | A | B | C | D |
|---|---|---|---|---|
| Excellent | Good | Satisfactory | Poor | |
|
Evidence base# |
Several level I or II studies with low risk of bias |
One or two level II studies with low risk of bias or a SR/multiple level III studies with low risk of bias |
Level III studies with low risk of bias, or level I or II studies with moderate risk of bias |
Level IV studies, or level I to III studies with high risk of bias |
|
Consistency* |
All studies consistent |
Most studies consistent and inconsistency may be explained |
Some inconsistency reflecting genuine uncertainty around clinical question |
Evidence is inconsistent |
|
Clinical impact |
Very large |
Substantial |
Moderate |
Slight or restricted |
|
Generalisability |
Population/s studied in body of evidence are the same as the target population for the guideline |
Population/s studied in body of evidence are similar to target population for the guideline |
Populations/s studied in body of evidence differ to target population for guideline but it is clinically sensible to apply this evidence to target population^ |
Populations/s studied in body of evidence differ to target population and hard to judge whether it is sensible to generalise to target population |
|
Applicability |
Directly applicable to Australian healthcare context |
Applicable to Australian healthcare context with few caveats |
Probably applicable to Australian healthcare context with some caveats |
Not applicable to Australian healthcare context |
#Level of evidence determined from the NHMRC evidence hierarchy
*If there is only one study, rank this component as ‘not applicable’
^For example, results in adults that are clinically sensible to apply to children OR psychosocial outcomes for one cancer that may be applicable to patients with another cancer
There is also capacity to note any other relevant factors that were considered by the guideline developers and the respective Working Group when judging the body of evidence and developing the wording of the recommendation.
The NHMRC grades given (A-D) are intended to indicate the strength of the body of evidence underpinning the recommendation (refer to Table 7). Grade A or B recommendations are generally based on a body of evidence that can be trusted to guide clinical practice, whereas Grades C or D recommendations must be applied cautiously to individual clinical and organisational circumstances and should be interpreted with care. A recommendation cannot be graded A or B unless evidence base and consistency of the evidence are both rated A and B respectively.8
Table 10: Definition of NHMRC grades of Recommendations8,9 (Note: This table is replicated in "Grading of Clinical Practice Guidelines")
| Grade of recommendation | Description |
|---|---|
|
A |
Body of evidence can be trusted to guide practice |
|
B |
Body of evidence can be trusted to guide practice in most situations |
|
C |
Body of evidence provides some support for recommendation(s) but care should be taken in its application |
|
D |
Body of evidence is weak and recommendation must be applied with caution |
By referring to the statements of evidence in combination with the NHMRC body of evidence matrix, a grade for each recommendation was derived from the respective grades allocated to the five key components. Grading the components of consistency, clinical impact, generalisability and applicability, was undertaken by the Working Group members, who discussed each section, and based on consensus achieved across the Working Group, arrived at these ratings.
The use of the NHMRC evidence hierarchy Table, categorises the respective study level according to the study design (refer to Table 8). This is used to determine the respective grades for evidence base and consistency of the recommendation.
Implementing the NHMRC Evidence Hierarchy, each included study in a systematic review should be assessed according to the following three dimensions of evidence:
- Strength of evidence (level of evidence, quality of evidence (risk of bias) and statistical precision.
- Size of effect (assessing the clinical importance of the findings of each study and hence addressing the clinical impact component of the body of evidence matrix.
- Relevance of evidence (translation of research evidence into clinical practice and is potentially the most subjective of the evidence assessments).
Table 11: NHMRC Evidence Hierarchy: designations of ‘levels of evidence’ according to type of research question9
| Level | Intervention | Diagnostic accuracy | Prognosis | Aetiology | Screening Intervention |
|---|---|---|---|---|---|
|
I |
A systematic review of level II studies |
A systematic review of level II studies |
A systematic review of level II studies |
A systematic review of level II studies |
A systematic review of level II studies |
|
II |
A randomised controlled trial |
A study of test accuracy with:
|
A prospective cohort study |
A prospective cohort study |
A randomised controlled trial |
|
III-1 |
A pseudo randomised controlled trial (i.e. alternate allocation or some other method) |
A study of test accuracy with:
|
All or none |
All or none |
A pseudo randomised controlled trial (i.e. alternate allocation or some other method) |
|
III-2 |
A comparative study with concurrent controls:
|
A comparison with reference standard that does not meet the criteria required for Level II and III-1 evidence |
Analysis of prognostic factors amongst persons in a single arm of a randomised controlled trial |
A retrospective cohort study |
A comparative study with concurrent controls:
|
|
III-3 |
A comparative study without concurrent controls:
|
Diagnostic case-control study |
A retrospective cohort study |
A case-control study |
A comparative study without concurrent controls:
|
|
IV |
Case series with either post-test or pre-test/post-test outcomes |
Study of diagnostic yield (no reference standard) |
Case series, or cohort study of persons at different stages of disease |
A cross-sectional study or case series |
Case series |
Appendix 2: Evidence Summaries for Grading the Recommendations
Appendix 2: Evidence Summaries for Grading the Recommendations Anonymous (not verified)Four Recommendations have been made based on the following key questions -
- What is the effectiveness of hypofractionated radiotherapy compared to conventionally fractionated radiotherapy for the treatment of early breast cancer?
In patients with early breast cancer who require post-operative whole breast radiotherapy who meet the following criteria:
- aged 50 years and over
- with pathological stage T1-2, N0, M0
- who have undergone breast conserving surgery, with clear surgical margins
Read more
- What is the effectiveness of hypofractionated radiotherapy compared to conventionally fractionated radiotherapy for the treatment of early breast cancer?
For patients who do not meet the selection criteria for Recommendation 1
Read more
- What is the optimal schedule for hypofractionated radiotherapy for the treatment of early breast cancer?
Read more
- What is the safety of hypofractionated radiotherapy compared to conventionally fractionated radiotherapy for the treatment of early breast cancer?
Read more
Recommendation 1
Recommendation 1 Anonymous (not verified)Question: What is the effectiveness of hypofractionated radiotherapy compared to conventionally fractionated radiotherapy for the treatment of early breast cancer?
In patients with early breast cancer who require post-operative whole breast radiotherapy who meet the following criteria:
- aged 50 years and over
- with pathological stage T1-2, N0, M0
- who have undergone breast conserving surgery, with clear surgical margins
|
1. Evidence base |
||
|
One level I study (meta-analysis of RCTs) with a low risk of bias and six level II (RCTs) studies with a low risk of bias |
A |
One or more level I studies with a low risk of bias or several level II studies with a low risk of bias |
|
B |
One or two Level II studies with a low risk of bias or SR/several Level III studies with a low risk of bias |
|
|
C |
One or two Level III studies with a low risk of bias or Level I or II studies with a moderate risk of bias |
|
|
D |
Level IV studies or Level I to III studies/SRs with a high risk of bias |
|
|
2. Consistency |
||
|
Studies reported equivalent outcomes (effectiveness and safety) for hypofractionated radiotherapy compared with standard radiotherapy |
A |
All studies consistent |
|
B |
Most studies consistent and inconsistency can be explained |
|
|
C |
Some inconsistency, reflecting genuine uncertainty around question |
|
|
D |
Evidence is inconsistent |
|
|
NA |
Not applicable (one study only) |
|
|
3. Clinical impact |
||
|
The demonstration of non-inferiority for hypofractionated radiotherapy was considered to represent a substantial clinical benefit from the patient’s perspective |
A |
Very large |
|
B |
Substantial |
|
|
C |
Moderate |
|
|
D |
Slight/Restricted |
|
|
4. Generalisability |
||
|
The evidence is based on data from a large number of patients with sufficient follow-up to be considered generalisable to the sub-population defined above |
A |
Evidence directly generalisable to target population |
|
B |
Evidence directly generalisable to target population with some caveats |
|
|
C |
Evidence not directly generalisable to the target population but could be sensibly applied |
|
|
D |
Evidence not directly generalisable to target population and hard to judge whether it is sensible to apply |
|
|
5. Applicability |
||
|
Key studies were conducted in settings considered to be similar to Australia (the UK, Canada and USA). |
A |
Evidence directly applicable to Australian healthcare context |
|
B |
Evidence applicable to Australian healthcare context with few caveats |
|
|
C |
Evidence probably applicable to Australian healthcare context with some caveats |
|
|
D |
Evidence not applicable to Australian healthcare context |
|
Other factors
Recent evidence indicates that tumour grade does not need to be taken into account when considering the use of hypofractionated radiotherapy. Consequently this characteristic has been removed from the selection criteria for the Recommendation and it was agreed that this update would be reflected in a corresponding Practice Point (a)
Evidence Statement Matrix
| Component | Rating | Description |
|---|---|---|
|
A |
One level I (meta-analysis of RCTs) and six level II (RCTs) studies with a low risk of bias |
|
A |
Studies reported equivalent outcomes for hypofractionated radiotherapy compared with standard radiotherapy |
|
B |
The demonstration of non-inferiority for hypofractionated radiotherapy was considered to represent a substantial clinical benefit from the patient’s perspective |
|
A |
The evidence is based on data from a large number of patients with sufficient follow-up to be considered generalisable to the target sub-population |
|
A |
Key studies were conducted in settings considered to be similar to Australia (the UK, Canada and USA). |
Recommendation
|
In selected patients* with early breast cancer who require post-operative whole breast radiotherapy, hypofractionated is a suitable alternative to conventionally fractionated radiotherapy, and should be offered where appropriate. *Patients
|
Grade Of Recommendation |
|
A |
Unresolved Issues
None
Implementation Of Recommendation
|
Will this recommendation result in changes in usual care? Yes, the updated recommendation is stronger (now Grade A) and broader (no longer limited by grade of tumour) than the previous recommendation. |
YES |
|
Are there any resource implications associated with implementing this recommendation? Yes, at the level of clinicians and service providers, implementation of this guideline may result in reduced waiting times due to an overall shorter treatment program and increased patient turnover. From the perspective of patients and their carers, increased uptake of hypofractionated radiotherapy would reduce the amount of time spent away from work and family, and might reduce out of pocket costs for patients travelling from non-metropolitan areas to receive radiotherapy. |
YES |
|
Will the implementation of this recommendation require changes in the way care is currently organised? No, hypofractionated radiotherapy schedules can already be delivered via current systems |
NO |
|
Are the guideline development group aware of any barriers to the implementation of this recommendation? |
NO |
Recommendation 2
Recommendation 2 Anonymous (not verified)Key question: What is the effectiveness of hypofractionated radiotherapy compared to conventionally fractionated radiotherapy for the treatment of early breast cancer?
- For patients who do not meet the selection criteria for Recommendation 1
|
1. Evidence base |
||
|
One level I study (meta-analysis of RCTs) with a low risk of bias and six level II (RCTs) studies with a low risk of bias |
A |
One or more level I studies with a low risk of bias or several level II studies with a low risk of bias |
|
B |
One or two Level II studies with a low risk of bias or SR/several Level III studies with a low risk of bias |
|
|
C |
One or two Level III studies with a low risk of bias or Level I or II studies with a moderate risk of bias |
|
|
D |
Level IV studies or Level I to III studies/SRs with a high risk of bias |
|
|
2. Consistency |
||
|
Some inconsistency which could not be explained due to the smaller number of patients in this sub-population included across the RCTs |
A |
All studies consistent |
|
B |
Most studies consistent and inconsistency can be explained |
|
|
C |
Some inconsistency, reflecting genuine uncertainty around question |
|
|
D |
Evidence is inconsistent |
|
|
NA |
Not applicable (one study only) |
|
|
3. Clinical impact |
||
|
Notwithstanding the smaller patient numbers, demonstration of non-inferiority for hypofractionated radiotherapy was considered to represent a substantial clinical benefit from the patient’s perspective |
A |
Very large |
|
B |
Substantial |
|
|
C |
Moderate |
|
|
D |
Slight/Restricted |
|
|
4. Generalisability |
||
|
The evidence is based on data from a moderate number of patients with sufficient follow-up to be considered generalisable to the sub-population defined above |
A |
Evidence directly generalisable to target population |
|
B |
Evidence directly generalisable to target population with some caveats |
|
|
C |
Evidence not directly generalisable to the target population but could be sensibly applied |
|
|
D |
Evidence not directly generalisable to target population and hard to judge whether it is sensible to apply |
|
|
5. Applicability |
||
|
Key studies were conducted in settings considered to be similar to Australia (the UK, Canada and USA). |
A |
Evidence directly applicable to Australian healthcare context |
|
B |
Evidence applicable to Australian healthcare context with few caveats |
|
|
C |
Evidence probably applicable to Australian healthcare context with some caveats |
|
|
D |
Evidence not applicable to Australian healthcare context |
|
Other factors
Longer follow-up and reporting of late effects may change the recommendation
Evidence Statement Matrix
| Component | Rating | Description |
|---|---|---|
|
A |
One level I study (meta-analysis of RCTs) with a low risk of bias and six level II (RCTs) studies with a low risk of bias |
|
C |
Some inconsistency which could not be explained due to the smaller number of patients in this sub-population included across the RCTs |
|
B |
Demonstration of non-inferiority for hypofractionated radiotherapy would represent a substantial clinical benefit |
|
A |
Sufficient follow-up from a moderate number of patients |
|
A |
Key studies were conducted in settings considered to be similar to Australia |
Recommendation
|
For women with early breast cancer who require post-operative whole breast radiotherapy and who are outside the selection criteria in Recommendation 1, hypofractionated radiotherapy could be considered as an alternative to conventionally fractionated radiotherapy. |
Grade Of Recommendation |
|
C |
Unresolved Issues
Due to the relatively small numbers of patients in sub-group analyses of randomised trials, there is more limited evidence to inform the safety and efficacy of hypofractionated radiotherapy for women:
- aged less than 50 years
- with locally advanced breast cancer
- with node positive disease
- who receive chemotherapy and/or targeted biological therapies.
Implementation Of Recommendation
|
Will this recommendation result in changes in usual care? No, this sub-population of patients will continue to receive either hypofractionated or conventionally fractionated radiotherapy based on clinical judgement |
NO |
|
Are there any resource implications associated with implementing this recommendation? No, this sub-population of patients will continue to receive standard care |
NO |
|
Will the implementation of this recommendation require changes in the way care is currently organised? |
NO |
|
Are the guideline development group aware of any barriers to the implementation of this recommendation? |
NO |
Recommendation 3
Recommendation 3 Anonymous (not verified)Key question: What is the optimal schedule for hypofractionated radiotherapy for the treatment of early breast cancer?
|
1. Evidence base |
||
|
Three level II studies (RCTs) with a low risk of bias |
A |
One or more level I studies with a low risk of bias or several level II studies with a low risk of bias |
|
B |
One or two Level II studies with a low risk of bias or SR/several Level III studies with a low risk of bias |
|
|
C |
One or two Level III studies with a low risk of bias or Level I or II studies with a moderate risk of bias |
|
|
D |
Level IV studies or Level I to III studies/SRs with a high risk of bias |
|
|
2. Consistency |
||
|
The START B RCT reported better outcomes for hypofractionated radiotherapy compared with standard radiotherapy. The Canadian RCT showed equivalent outcomes for hypofractionated radiotherapy and standard radiotherapy. The Spooner RCT showed comparable rates of tumour control and radiation therapy effects. |
A |
All studies consistent |
|
B |
Most studies consistent and inconsistency can be explained |
|
|
C |
Some inconsistency, reflecting genuine uncertainty around question |
|
|
D |
Evidence is inconsistent |
|
|
NA |
Not applicable (one study only) |
|
|
3. Clinical impact |
||
|
Demonstration of non-inferiority for hypofractionated radiotherapy represents a substantial clinical benefit |
A |
Very large |
|
B |
Substantial |
|
|
C |
Moderate |
|
|
D |
Slight/Restricted |
|
|
4. Generalisability |
||
|
Sufficient follow-up from a large number of patients |
A |
Evidence directly generalisable to target population |
|
B |
Evidence directly generalisable to target population with some caveats |
|
|
C |
Evidence not directly generalisable to the target population but could be sensibly applied |
|
|
D |
Evidence not directly generalisable to target population and hard to judge whether it is sensible to apply |
|
|
5. Applicability |
||
|
Key studies were conducted in settings considered to be similar to Australia |
A |
Evidence directly applicable to Australian healthcare context |
|
B |
Evidence applicable to Australian healthcare context with few caveats |
|
|
C |
Evidence probably applicable to Australian healthcare context with some caveats |
|
|
D |
Evidence not applicable to Australian healthcare context |
|
Other Factors
It was noted that the proportion of women receiving tumour bed boost was similar among the treatment groups in START B and Spooner, and that a post hoc subgroup analysis demonstrated no statistically significant difference in local-regional relapse rates of cosmetic outcomes between hypofractionated and conventionally fractionated radiotherapy in patients who received tumour bed boost versus those who did not. It was agreed that Practice Point (b) be included to provide guidance on the optimal schedule for a tumour bed boost, based on the schedule used in one of the RCTs included for this Question (START B).
Evidence Statement Matrix
| Component | Rating | Description |
|---|---|---|
|
A |
Three level II studies (RCTs) with a low risk of bias |
|
B |
START B reported better outcomes for hypofractionated radiotherapy compared with standard radiotherapy. The Canadian and Spooner trials showed equivalent outcomes for hypofractionated radiotherapy and standard radiotherapy. |
|
B |
Demonstration of non-inferiority for hypofractionated radiotherapy would represent a substantial clinical benefit |
|
A |
Sufficient follow-up from a large number of patients |
|
A |
Key studies were conducted in settings considered to be similar to Australia |
Recommendation
|
For patients not receiving a tumour bed boost, recommended hypofractionated schedules for whole breast radiotherapy based on current evidence are:
|
Grade Of Recommendation |
|
A |
Unresolved Issues
None
Implementation Of Recommendation
|
Will this recommendation result in changes in usual care? Yes, hypofractionated radiotherapy, with or without tumour bed boost, can now be considered as a treatment option |
YES |
|
Are there any resource implications associated with implementing this recommendation? Reduced waiting times due to shorter treatment program and increased patient turnover |
YES |
|
Will the implementation of this recommendation require changes in the way care is currently organised? No, hypofractionated radiotherapy schedules can already be delivered via current systems |
NO |
|
Are the guideline development group aware of any barriers to the implementation of this recommendation? |
NO |
Recommendation 4
Recommendation 4 Anonymous (not verified)Key question: What is the safety of hypofractionated radiotherapy compared to conventionally fractionated radiotherapy for the treatment of early breast cancer?
|
1. Evidence base |
||
|
One level 1 (meta-analysis of RCTs) and six phase II (RCTs) studies with a low risk of bias |
A |
One or more level I studies with a low risk of bias or several level II studies with a low risk of bias |
|
B |
One or two Level II studies with a low risk of bias or SR/several Level III studies with a low risk of bias |
|
|
C |
One or two Level III studies with a low risk of bias or Level I or II studies with a moderate risk of bias |
|
|
D |
Level IV studies or Level I to III studies/SRs with a high risk of bias |
|
|
2. Consistency |
||
|
Studies reported different adverse events. Across the adverse events studies reported no difference, a difference that favoured hypofractionated radiotherapy, or a difference that favoured conventionally fractionated radiotherapy. On balance the adverse event profile for hypofractionated versus conventionally fractionated radiotherapy were comparable. |
A |
All studies consistent |
|
B |
Most studies consistent and inconsistency can be explained |
|
|
C |
Some inconsistency, reflecting genuine uncertainty around question |
|
|
D |
Evidence is inconsistent |
|
|
NA |
Not applicable (one study only) |
|
|
3. Clinical impact |
||
|
The comparability of the adverse event profiles with the different fractionation methods was considered to be of significance. |
A |
Very large |
|
B |
Substantial |
|
|
C |
Moderate |
|
|
D |
Slight/Restricted |
|
|
4. Generalisability |
||
|
Whilst the studies are broadly generalisable, some uncertainty remains regarding the possible cardiac effects of hypofractionated radiotherapy in women with left-sided tumours especially as women with pre-existing heart disease were under-represented in the studies |
A |
Evidence directly generalisable to target population |
|
B |
Evidence directly generalisable to target population with some caveats |
|
|
C |
Evidence not directly generalisable to the target population but could be sensibly applied |
|
|
D |
Evidence not directly generalisable to target population and hard to judge whether it is sensible to apply |
|
|
5. Applicability (Is the body of evidence relevant to the Australian healthcare context in terms of health services/delivery of care and cultural factors?) |
||
|
Key studies were conducted in settings considered to be similar to Australia |
A |
Evidence directly applicable to Australian healthcare context |
|
B |
Evidence applicable to Australian healthcare context with few caveats |
|
|
C |
Evidence probably applicable to Australian healthcare context with some caveats |
|
|
D |
Evidence not applicable to Australian healthcare context |
|
Other Factors
Concerns were raised regarding the possibility of late effects on the heart for women with left-sided tumours. A supplementary review of the literature was undertaken to address these concerns around cardiotoxicity. Whilst it was recognised that late cardiac effects may take up to 20 years to develop, the current evidence base shows no difference in longer term cardiac mortality or morbidity between hypofractionated and conventionally fractioned schedules. It was agreed that whilst a left-sided tumour should not be included as an exclusion criterion for hypofractionated radiotherapy, a Practice Point (c) would be included to highlight the importance of adopting heart sparing protocols, particularly in women with pre-existing heart disease, who are under-represented in the studies in the evidence base.
Evidence Statement Matrix
| Component | Rating | Description |
|---|---|---|
|
A |
One level 1 (meta-analysis of RCTs) and six phase II (RCTs) studies with a low risk of bias |
|
B |
On balance the adverse event profile for hypofractionated versus conventionally fractionated radiotherapy were comparable. |
|
B |
The comparability of the adverse event profiles was considered to be of substantial clinical impact. |
|
B |
Patients broadly generalisable, but with under-representation of women with heart disease |
|
A |
Key studies were conducted in settings considered to be similar to Australia |
Recommendation
| When selecting an appropriate radiotherapy schedule, consideration should be given to the possibility of adverse events including acute reactions and late effects. | Grade Of Recommendation |
|
B |
Unresolved Issues
The longest duration of follow-up from RCTs is 9.3 and 9.7 years (START A and B), and from prospective cohort studies is 13-14 years. Ideal follow-up for late cardiac effects is thought to be 15-20 years, so additional follow-up for late effects is still required.
Implementation Of Recommendation
|
Will this recommendation result in changes in usual care? |
NO |
|
Are there any resource implications associated with implementing this recommendation? |
NO |
|
Will the implementation of this recommendation require changes in the way care is currently organised? |
NO |
|
Are the guideline development group aware of any barriers to the implementation of this recommendation? |
NO |