Cancer clinical trials are part of a broader drug development pathway, during which new drugs are identified, tested and approved for clinical use.
The drug development pathway comprises 5 main stages:
- discovery
- pre-clinical
- clinical (which comprises Phase 1, 2 and 3 trials)
- registration and marketing approval
- post-marketing.
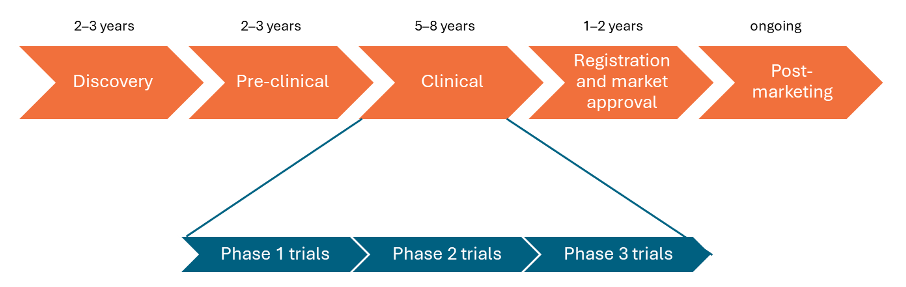
The drug development pathway can take 10–20 years. It is expensive and risky, because out of 5,000–10,000 potential drugs discovered, only 1 or 2 may end up being developed into a new treatment.
For information about cancer in general and its treatments, see Impacted by cancer.
Discovery
The discovery stage refers to laboratory research where researchers try to understand the properties of different biological agents, how they work and how they might be directed against different diseases.
Researchers usually study a particular molecular pathway in cell development, maintenance or death. They will try to understand how it works, or why it goes wrong and how specific treatments might interrupt the pathway or the mechanism that is out of control.
The discovery stage will usually involve in vitro research. In vitro literally means ‘in glass’, so these studies do not involve animals or humans. In in vitro studies, biological agents are mixed with different cancer cell lines to see whether they affect cell development. If the cells stop growing or die, it is a signal that the treatment is doing something and therefore is worth investigating further.
Pre-clinical
In the pre-clinical stage, treatments that have shown some activity in the discovery phase are tested further. Pre-clinical studies may include more in vitro studies, but will also include in vivo animal studies.
Pre-clinical research aims to better understand better how the intervention works, potential side effects, and whether these side effects are acceptable or not. This is called ‘nonclinical safety’. Researchers can only move onto clinical trials after they have shown acceptable nonclinical safety of treatments.
Clinical
The clinical stage is when interventions are tested in people, called clinical trials. This is the longest stage of all 5 drug development stages.
Research in this stage further assesses if the drug is safe and effective. Clinical trials are further split into Phase 1, 2 and 3 trials.
Phase 1 clinical trials
Phase 1 trials are the first trials in the life of a new drug or treatment to find out:
- the safe dose range, often called the maximum tolerated dose
- the side effects
- how the body copes with the drug
- if the drug works the way it did in the pre-clinical phase.
Phase 1 clinical trials are usually small, with up to 30 people involved. These trials involve people with cancer who have no other treatment options. This is different to drug trials for other conditions, where Phase 1 trials often involve healthy volunteers.
Phase 2 trials
Phase 2 trials aim to find out:
- if the new drug works well enough to test in a larger group of people
- which types of cancer the treatment works for
- more about side effects and how to manage them
- more about the best dose to use.
Phase 2 trials are trials that involve people who have the same type of cancer, or with several different types of cancer. There may be up to 100 people taking part in a phase 2 trial.
Phase 3 trials
Phase 3 trials are trials that compare:
- new treatments with the best currently available treatment, also known as standard care
- different doses of ways of giving the standard treatment
- a new therapy schedule with a standard one.
Phase 3 cancer trials are usually aimed at determining whether a particular drug improves the overall survival of people with cancer.
Patients are usually randomised to one of 2 groups (or ‘arms’):
- intervention (the drug being tested)
- control (placebo or standard of care).
Such trials are also known as randomised controlled trials and involve hundreds of patients.
Registration review and marketing approval
The registration review and marketing approval stage is where all the results generated in the pre-clinical and clinical development phases are submitted to the appropriate regulatory bodies. In Australia, this is the Therapeutic Goods Administration (TGA). In the US, this is the Food and Drug Administration (FDA).
The results are evaluated to determine whether the new drug should be registered on the Australian Register of Therapeutic Goods (ARTG). In Australia, doctors can only prescribe a drug if it is listed on the ARTG. Note that this is not the same as when a treatment becomes government-funded through the Pharmaceutical Benefit Scheme (PBS). This requires another process.
Post-marketing
The post-marketing stage is when more studies are done after a treatment is commercially available. These studies aim to understand how best to use the approved drug in the most cost-effective way for the patients and for the broader community. Sometimes, this stage is referred to as Phase 4 clinical trials.




